Abstract
Purpose
The main objective of the present study was the investigation of possible influence of lens opacification on macular pigment optical density (MPOD) measurements.
Methods
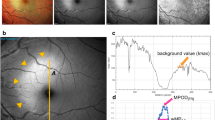
Eighty-six eyes of 64 patients (mean age 73.4 ± 8.3 years) were included in the study. MPOD was prospectively measured using the one-wavelength reflection method (Visucam500, Carl Zeiss Meditec AG) before and after cataract extraction, with implantation of a blue-light filtering intraocular lens (AlconSN60WF). The median of the maximum optical density (MaxOD) and the median of the mean optical density (MeanOD) measurements of macular pigment across the subject group were evaluated.
Results
Statistically significant differences were noticed between pre-operative and post-operative measurements, the absolute values were generally lower after cataract extraction. The following median (lower/upper quartile) differences across the group were determined: MaxOD −33.8 % (−46.2 to −19.1 %), MeanOD −44.0 % (−54.6 to −26.6 %). Larger changes were observed in elderly patients [<70 years of age (n = 25 eyes): MaxOD −13.4 % (−20.5 to 3.6 %), MeanOD −23.6 % (−30.5 to −15.3 %) versus patients ≥70 years (n = 61 eyes) MaxOD −40.5 % (−53.2 to −30.1 %), MeanOD −47.2 % (−57.8 to −40.1 %)] and in patients with progressed stage of cataract. MaxOD for lens opacification grade 1 (n = 9 eyes): −27.4 % (−42.1 to −19.6 %), grade 2 (n = 26 eyes): −35.0 % (−44.2 to −25.3 %), grade 3 (n = 21 eyes): −34.4 % (−45.4 to −11.4 %), grade 4 (n = 25 eyes): −32.6 % (−53.2 to −6.4 %), and grade 5 (n = 5 eyes): −53.5 % (−61.7 to −38.7 %) and MeanOD for cataract stage 1 (n = 9 eyes): −42.6 % (−46.0 to −26.0 %), stage 2 (n = 26 eyes): −44.1 % (−51.8 to −26.2 %), stage 3 (n = 21 eyes): −45.7 % (−54.7 to −24.7 %), stage 4 (n = 25 eyes): −39.5 % (−59.4 to −26.1 %), and stage 5 (n = 5 eyes): −57.0 % (−66.1 to −51.4 %).
Conclusions
As established by comparison of pre- to post-operative measurements, cataract presented a strong effect on MPOD measured by one-wavelength reflection method. Particular care should therefore be taken when evaluating MPOD using this method in elderly patients with progressed stage of cataract. Future optimization of correcting parameters of scattered light and consideration of cataract influence may allow more precise evaluation of MPOD.





Similar content being viewed by others
References
Snodderly DM, Auran JD, Delory FC (1984) The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis 25:674–685
Snodderly DM, Brown PK, Delori FC, Auran JD (1984) The macular pigment. I. absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci 25:660–673
Sommerburg OG, Siems WG, Hurst JS, Lewis JW, Kliger DS, van Kuijk FJ (1999) Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr Eye Res 19:491–495
Liew SHT, Clare EG, Spector TD, Mellerio J, Van Kuijk FJ, Beatty S, Fitzke F, Marshall J, Hammond CJ (2005) Central retinal thickness is positively correlated with MPOD. Exp Eye Res 82:915–920
Krinsky NI, Landrum JT, Bone RA (2003) Biologic mechanisms of the protective role of lutein and zeaxanzhin in the eye. Annu Rev Nutr 23:171–201
Hirsch J, Curcio CA (1989) The spatial resolution capacity of human foveal retina. Vision Res 29:1095–1101
Hammond BR Jr, Wooten BR (2005) CFF thresholds: relation to macular pigment optical density. Ophthalmic Physiol Opt 25:315–319
Kvansakul J, Rodriguez-Carmona M, Edgar DF, Barker FM, Köpcke W, Schalch W, Barbur JL (2006) Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmol Physiol Opt 26:362–371
Rodriguez-Carmona M, Kvansakul J, Harlow JA, Köpcke W, Schalch W, Barbur JL (2006) The effects of supplementation with lutein and/or zeaxanthin on human macular pigment density and colour vision. Ophthal Physiol Opt 26:137–147
Stringham JM, Hammond BR Jr (2007) The glare hypothesis of macular pigment function. Optom Vis Sci 84:859–864
Berrow EJ, Bartlett HE, Eperjesi F, Gibson JM (2013) The effects of a lutein-based supplement on objective and subjective measures of retinal and visual function in eyes with age-related maculopathy—a randomised controlled trial. Br J Nutr 109:2008–2014
Nolan JM, Loughman J, Akkali MC, Stack J, Scanlon G, Davison P, Beatty S (2011) The impact of MP augmentation on visual performance in normal subjects: COMPASS. Vision Res 51:459–469
Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB (2001) Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res 72:215–223
Reading VM, Weale RA (1974) Macular pigment and chromatic aberration. J Opt Soc Am 64:231–234
Kirshfeld K (1982) Carotenoid pigments: their possible role in protecting against photooxidation in eyes and photoreceptor cells. Proc Res Soc Lond B 216:71–85
Putnam CM, Kinerk WT, Bassi CJ (2013) Central serous chorioretinopathy produces macular pigment profile changes. Optom Vis Sci 90:206–212
Nolan JM, Stack J, O’Donovan O, Loane E, Beatty S (2007) Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Exp Eye Res 84:61–74
Helb HM, Charbel Issa P, van der Veet RLP, Berendschot TTJM, Scholl HPN, Holz FG (2008) Abnormal macular pigment distribution in type 2 idiopathic macular telangiectasia. Retina 28:808–816
Loane E, Kelliher C, Beatty S, Nolan JM (2008) The rationale and evidence base for a protective role of macular pigment in age-related maculopathy. Br J Ophthalmol 92:1163–1168
Beatty S, Murray IJ, Henson DB, Carden D, Koh HH, Boulton ME (2001) Macular pigment and risk for age-related macular degeneration in subjects from a northern European population. Invest Ophthalmol Vis Sci 42:439–446
Bernstein PS, Zhao DY, Wintch SW, Ermakov IV, McClane RW, Gellermann W (2002) Resonance Raman measurement of macular carotenoids in normal subjects and in ARMD patients. Ophthalmology 109:1780–1787
Kaya S, Weigert G, Pemp B, Sacu S, Werkmeister RM, Dragostinoff N, Garhöfer G, Schmidt-Erfurth U, Schmetterer L (2012) Comparison of macular pigment in patients with age-related macular degeneration and healthy control subjects—a study using spectral fundus reflectance. Acta Ophthalmol 90:e399–e403
Wüstemeyer H, Jahn C, Nestler A, Barth T, Wolf S (2002) A new instrument for the quantification of macular pigment density: first results in patients with AMD and healthy subjects. Graefes Arch Clin Exp Ophthalmol 240:666–671
Hammond BR Jr, Johnson EJ, Russell RM, Krinsky NI, Yem KJ, Edwards RB, Snodderly DM (1997) Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci 38:1795–1801
Leung IY (2008) Macular pigment: new clinical methods of detection and the role of carotenoids in atrophic age-related macular degeneration. Optometry 79:266–272
Berendschot TT, Goldbohm RA, Klöpping WA, van de Kraats J, van Norel J, van Norren D (2000) Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Invest Ophthalmol Vis Sci 41:3322–3326
Bone RA, Landrum W, Guerra LH, Ruiz CA (2003) Lutein and zeaxanthin dietary supplements raise MPD and serum concentrations of these carotenoids im humans. J Nutr 133:992–998
Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J (2004) Double-masked, placebo controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic ARMD: the veterans last Study (lutein antioxidant supplementation trial). Optometry 75:216–230
Dawczynski J, Jentsch S, Schweitzer D, Hammer M, Lang GE, Strobel J (2013) Long term effects of lutein, zeaxanthin and omega-3 LCPUFAs supplementation on optical density of macular pigment in AMD patients: the LUTEGA study. Graefes Arch Clin Exp Ophthalmol 251(12):2711-2723. doi:10.1007/s00417-013-2376-6
Gale CR, Hall NF, Phillips DI (2003) Lutein and zeaxanthin status and risk of atrophic age-related macular degeneration. Invest Ophthalmol Vis Sci 44:2461–2465
Yonova-Doing E, Hysi PG, Venturini C, Williams KM, Nag A, Beatty S, Liew SH, Gilbert CE, Hammond CJ (2013) Candidate gene study of macular response to supplemental lutein and zeaxanthin. Exp Eye Res 115:172–177
Trieschmann M, Beatty S, Nolan JM, Hense HW, Heimes B, Austermann U, Fobker M, Pauleikhoff D (2007) Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. Exp Eye Res 84:718–728
Dawczynski J, Jentsch S, Schweitzer D, Hammer M, Strobel J (2012) Changes of macular pigment and drusen morphology in patients with lutein supplementation. Klein Monatsbl Augenheilkd 229:69–71
Bernstein PS, Delori FC, Richer S, van Kuijk FJ, Wenzel AJ (2009) The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vision Res 50:716–728
Howells O, Eperjesi F, Bartlett H (2011) Measuring macular pigment optical density in vivo:a review of techniques. Graefes Arch Clin Exp Ophthalmol 249:315–347
Delori FC (1994) Spectrophotometer for noninvasive measurement of intrinsic fluorescence and reflectance of the ocular fundus. Appl Opt 33:7439–7452
Delori FC (2004) Autofluorescence method to measure macular pigment optical densities fluorometry and autofluorescence imaging. Arch Biochem Biophys 430:156–162
Rougier MB, Delyfer MN, Korobelnik JF (2008) Measuring macular pigment in vivo. J Fr Ophthalmol 31:445–453
Bernstein PS, Sharifzadeh M, Liu A, Ermakov I, Nelson K, Sheng X, Panish C, Carlstrom B, Hoffmann RO, Gellermann W (2013) Blue-light reflectance imaging of macular pigment in infants and children. Invest Ophthalmol Vis Sci 54:4034–4040
Van de Kraats J, Berendschot T, van Norren D (1996) The pathways of light measured in fundus reflectometry. Vision Res 36:2229–2247
Van de Kraats J, Berendschot T, Valen S, van Norren D (2006) Fast assessment of the central macular pigment density with natural pupil using the macular pigment reflectometer. J Biomed Opt 11:064031. doi:10.1117/1.2398925
Schweitzer D, Jentsch S, Dawczynski J, Hammer M, Wolf S, Wolf-Schnurrbusch U (2010) Simple and objective method for routine detection of the macular pigment xanthophyll. J Biomed Opt 15:061714. doi:10.1117/1.3526358
Van den Berg TJ, Ijspeert JK, de Waard PW (1991) Dependence of intraocular stray light on pigmentation and light transmission through the ocular wall. Vision Res 31:1361–1367
Artigas JM, Felipe A, Navea A, Fandiño A, Artigas C (2012) Spectral transmission of the human crystalline lens in adult and elderly persons: color and total transmission of visible light. Invest Ophthalmol Vis Sci 53:4076–4084
Terade H, Sawa M, Akiba J, Ueno N, Chakrabarti B (1994) Spectral transmittance of normal human crystalline lens. Nihon Ganka Gakkai Zasshi 98:1101–1108
Van den Berg TJTP, van Rijn LJ, Michael R, Heine C, Coeckelbergh T, Nischler C, Wilhelm H, Grabner G, Emesz M, Barraquer RI, Coppens JE, Franssen L (2007) Straylight effects with aging and lens extraction. Ophthalmol 144:358–363
Van den Berg TJTP, Ijspeert JK (1994) Light scattering in donor lenses. Vision Res 35:169–177
Van de Kraats J, van Norren D (2007) Optical density of the aging human ocular media in the visible and the UV. J Opt Soc Am 24:1842–1857
Sasamoto Y, Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Nishida K (2011) Effect of cataract in evaluation of macular pigment optical density by autofluorescence spectrometry. Invest Ophthalmol Vis Sci 52:927–932
Delori FC, Goger DG, Dorey CK (2001) Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci 42:1855–1866
Van den Berg TJ, Felius J (1995) Relationship between spectral transmittance and slit lamp color of human lenses. Invest Ophthalmol Vis Sci 36:322–329
Boettner EA, Reimer WJ (1962) Transmission of the ocular media. Invest Ophthalmol Vis Sci 1:776–783
Algvere PV, Torstensson PA, Tengroth BM (1993) Light transmittance of ocular media in living rabbit eyes. Invest Ophthalmol Vis Sci 34:349–354
Bernstein PS, Yoshida MD, Katz NB, McClane RW, Gellermann W (1998) Raman detection of macular carotenoid pigments in intact human retina. Invest Ophthalmol Vis Sci 39:2003–2011
Gellermann W, Ermakov IV, Ermakova MR, McClane RW, Zhao DY, Bernstein PS (2002) In vivo resonant Raman measurement of macular carotenoid pigments in the young and the aging human retina. J Opt Soc Am A 19:1172–1186
Ciulla TA, Hammond BR Jr, Yung CY, Linda M, Pratt LM (2001) Macular pigment optical density before and after cataract extraction. Invest Ophthalmol Vis Sci 42:1338–1341
Berendschot TT, van Norren D (2005) On the age dependency of the macular pigment optical density. Exp Eye Res 81:602–609
Loan JM, Stack J, O’Donavan O, Loan E, Betty S (2007) Risk factors for age-related maculopathy are associated with a lack of macular pigment. Exp Eye Res 84:61–74
Ciulla TA, Hammond BR Jr (2004) Macular pigment density and aging, assessed in the normal elderly and those with cataracts and age-related macular degeneration. Am J Ophthalmol 138:582–587
Delori FC, Goger DG, Hammond BR, Snodderly DM, Burns SA (2001) Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. J Opt Soc Am A Opt Image Sci Vis 18:1212–1230
Berendschot TT, Broekmans WM, Klopping-Ketelaar IA, Kardinaal AF, van Poppel G, van Norren D (2002) Lens aging in relation to nutritional determinants and possible risk factors for age-related cataract. Arch Ophthalmol 120:1732–1737
Werner JS, Donnelly SK, Kliegl R (1987) Aging and human macular pigment density. Appended with translations from the work of Max Schultze and Ewald Hering. Vision Res 27:257–268
Bone RA, Landrum JT, Fernandez L, Tarsis SL (1988) Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci 29:843–849
Bernstein PS, Zhao DY, Sharifzadeh M, Ermakov IV, Gellermann W (2004) Resonance Raman measurement of macular carotenoids in the living human eye. Arch Biochem Biophys 430:163–169
Berendschot TT, van Norren D (2004) Objective determination of the macular pigment optical density using Fundus reflectance spectroscopy. Arch Biochem Biophys 430:149–155
Wooten BR, Hammond BR Jr (2005) Spectral absorbance and spatial distribution of MP using heterochromatic flicker photometry. Optom Vis Sci 82:378–386
Lam RF, Rao SK, Fan DS, Lau FT, Lam DS (2005) Macular pigment optical density in a Chinese sample. Curr Eye Res 30:799–805
Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME (2001) Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci 42:439–446
Nolan J, O’Donovan O, Kavanagh H, Stack J, Harrison M, Muldoon A, Mellerio J, Beatty S (2004) Macular pigment and percentage of body fat. Invest Ophthalmol Vis Sci 45:3940–3950
Hammond BR Jr, Caruso-Avery M (2000) Macular pigment optical density in a Southwestern sample. Invest Ophthalmol Vis Sci 41:1492–1497
Obana A, Hiramitsu T, Gohto Y, Ohira A, Mizuno S, Hirano T, Bernstein PS, Fujii H, Iseki K, Tanito M, Hotta Y (2008) Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology 115:147–157
Chen SF, Chang Y, Wu JC (2001) The spatial distribution of macular pigment in humans. Curr Eye Res 23:422–434
Mellerio J, Ahmadi-Lari S, van Kuijk F, Pauleikhoff D, Bird A, Marshall J (2002) A portable instrument for measuring macular pigment with central fixation. Curr Eye Res 25:37–47
Nolan JM, O’Reilly P, Loughman J, Stack J, Loane E, Connolly E, Beatty S (2009) Augmentation of macular pigment following implantation of blue light–filtering intraocular lenses at the time of cataract surgery. Invest Ophthalmol Vis Sci 50:4777–4785
Demirel S, Bilici S, Batıoglu F, Ozmert E (2014) The effect of age and cataract surgery on macular pigment optic density: a cross-sectional, comparative study. Graefes Arch Clin Exp Ophthalmol 252(2):213–218. doi:10.1007/s00417-013-2424-2
Johnson CA, Adams AJ, Twelker JD, Quigg JM (1988) Age-related changes in the central visual field for short wavelength-sensitive pathways. J Opt Soc Am A 5:2131–2139
Acknowledgments
The authors extend their sincere thanks to all the patients who volunteered their service to make this study possible.
Conflict of interest
None of the authors has any financial interest in the presented study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Komar, B., Rauscher, F.G., Wiedemann, R. et al. Macular pigment optical density measurements by one-wavelength reflection photometry—influence of cataract surgery on the measurement results. Graefes Arch Clin Exp Ophthalmol 252, 1717–1727 (2014). https://doi.org/10.1007/s00417-014-2627-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2627-1