Abstract
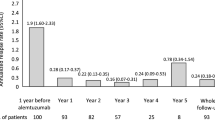
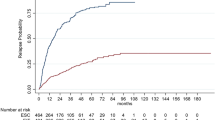
Performing a therapeutic switch in MS is still a matter of debate. Objective of our study is to compare switching to another first-line therapy with switching to a second-line therapy in persons with relapsing-remitting multiple sclerosis (pwRRMS). A retrospective analysis of data prospectively collected was performed. PwRRMS experiencing on-treatment disease activity were included. No clinical relapse, no sustained disability progression by the Expanded Disability Status Scale (EDSS), and no radiological activity (new T2 and/or gadolinium-enhanced brain lesions) were used as indicators of no disease activity (NEDA 3). Time to reach the first relapse after switch and time to reach an EDSS of 4.0 were also evaluated. Ninety-one pwRRMS were enrolled. Forty-eight (52.7 %) were on lateral switch, and 43 (47.3 %) on escalation switch. At baseline, the two groups differed for T2 and T1 brain lesions number (higher in the escalation group, p < 0.005). The proportion of pwRRMS who were NEDA 3 after 24 months from the switch was similar in the two groups (20.8 % in lateral group and 18.6 % in escalation group). No difference in timing to reach the first relapse after switch and an EDSS of 4.0 were found. Therefore, in selected pwRRMS, lateral and escalation strategies showed similar efficacy in delaying MS progression.





Similar content being viewed by others
References
Ingwersen J, Aktas O, Hartung HP (2016) Advances in and algorithms for the treatment of relapsing-remitting multiple sclerosis. Neurotherapeutics 13(1):47–57
Dörr J, Paul F (2015) The transition from first-line to second-line therapy in multiple sclerosis. Curr Treat Options Neurol 17(6):354
Gajofatto A, Benedetti MD (2015) Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin Cases 3(7):545–555
Derfuss T (2012) Personalized medicine in multiple sclerosis: hope or reality? BMC Med 10:116
Zaffaroni M, Rizzo A, Baldini SM, Ghezzi A, Comi G (2008) Induction and add-on therapy with mitoxantrone and interferon beta in multiple sclerosis. Neurol Sci 29(Suppl 2):S230–S232
Comi G (2008) Induction vs. escalating therapy in multiple sclerosis: practical implications. Neurol Sci 29(Suppl 2):S253–S255
What is MSBase. MSBase registry website. Available from: https://www.msbase.org. Accessed April 2016
Poser CM, Paty DW, Scheinberg L et al (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13:227–231
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50:121–127
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1452
Havrdova E, Galetta S, Stefoski D, Comi G (2012) Freedom from disease activity in multiple sclerosis. Neurology 74(suppl 3):S3–S7
Outteryck O (2016) Natalizumab in relapsing-remitting multiple sclerosis. Expert Rev Neurother. (Epub ahead of print). Accessed April 2016
Barbin L, Rousseau C, Jousset N et al (2016) Comparative efficacy of fingolimod vs natalizumab: a French multicenter observational study. Neurology 86(8):771
Bertolotto A, Capobianco M, Amato MP, Capello E, Capra R, Centonze D (2014) Guidelines on the clinical use for the detection of neutralizing antibodies (NAbs) to IFN beta in multiple sclerosis therapy: report from the Italian Multiple Sclerosis Study group. Neurol Sci 35(2):307–316
Río J, Tintoré M, Sastre-Garriga J et al (2012) Change in the clinical activity of multiple sclerosis after treatment switch for suboptimal response. Eur J Neurol Off J Eur Fed Neurol Soc 19:899–904
Caon C, Din M, Ching W, Tselis A, Lisak R, Khan O (2006) Clinical course after change of immunomodulating therapy in relapsing-remitting multiple sclerosis. Eur J Neurol Off J Eur Fed Neurol Soc 13:471–474
Prosperini L, Borriello G, De Giglio L, Leonardi L, Barletta V, Pozzilli C (2011) Management of breakthrough disease in patients with multiple sclerosis: when an increasing of Interferon beta dose should be effective? BMC Neurol 11:26. Accessed April 2016
D’Amico E, Leone C, Caserta C, Patti F (2015) Oral drugs in multiple sclerosis therapy: an overview and a critical appraisal. Expert Rev Neurother 15(7):803–824
Fox RJ, Miller DH, Phillips JT et al (2012) On behalf of the CONFIRM Study Investigators. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 367:1087–1097
Vermersch P, Czlonkowska A, Grimaldi LM et al (2014) Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler 20(6):705–716
Spelmann T, Kalincik Thomas, Zhang Annie et al (2015) Comparative efficacy of switching to natalizumab in active multiple sclerosis. Ann Clin Transl Neurol. doi:10.1002/acn3.180
Khatri B, Barkhof F, Comi G, Hartung HP, Kappos L, Montalban X (2009) TRANSFORMS study group. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol 10(6):520–529
Coles AJ, Compston DA, Selmaj KW et al (2008) Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. CAMMS223 trial investigators. N Engl J Med 359(17):1786–1801
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP (2012) CARE-MS I investigators Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380(9856):1819–1828
Coles JA, Twyman CL, Arnold DL et al (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial, CARE-MS II investigators. Lancet 380(9856):1829–1839
Kappos L, Wiendl H, Selmaj K et al (2015) Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. J N Engl J Med 373(15):1418–1428
A study of ocrelizumab in comparison with interferon beta 1a (Rebif) in patients with relapsing multiple sclerosis clinicaltrials.gov. http://clinicaltrials.gov/ct2/show/NCT01412333. Accessed 20 Mar 2016
A study of ocrelizumab in comparison with interferon beta 1a (Rebif) in patients with relapsing multiple sclerosis clinicaltrials.gov. http://clinicaltrials.gov/ct2/show/NCT01247324. Accessed 20 Mar 2016
AAN (2016) Efficacy and safety of ocrelizumab in primary progressive multiple sclerosis: results of the phase III double-blind, placebo-controlled ORATORIO study. Vancouver S49.001 (oral), Thursday, 21 April, 1:00 p.m. PDT
Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A (2012) Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 366(20):1870–1880
Reynolds MW, Stephen R, Seaman C, Rajagopalan K (2010) Healthcare resource utilization following switch or discontinuation in multiple sclerosis patients on disease modifying drugs. J Med Econ 13:90–98
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Patti served on the scientific advisory board for Teva, Biogen-Idec, Bayer-Schering, Novartis, and has received honoraria as a speaker for Teva, Biogen, Merck-Serono, Bayer-Schering, Genzyme/Sanofi, and Novartis. Dr. D’Amico received funding travel by Teva, Biogen, Merck-Serono, Bayer-Schering, Genzyme/Sanofi, Novartis. The other authors have nothing to disclose.
Ethical standards
Manuscript complains with ethical standards; it has been approved by ethics committee and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
All the patients have given their informed consent prior to their inclusion in the study. Details that might disclose their identity have been omitted.
Rights and permissions
About this article
Cite this article
D’Amico, E., Leone, C., Zanghì, A. et al. Lateral and escalation therapy in relapsing-remitting multiple sclerosis: a comparative study. J Neurol 263, 1802–1809 (2016). https://doi.org/10.1007/s00415-016-8207-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8207-z