Abstract
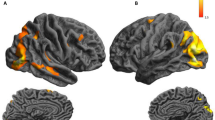
Posterior cortical atrophy (PCA) is a neurodegenerative syndrome in which the most pronounced pathologic involvement is in the occipito-parietal visual regions. Herein, we aimed to better define the cortical reflection of this unique syndrome using a thorough battery of behavioral and functional MRI (fMRI) tests. Eight PCA patients underwent extensive testing to map their visual deficits. Assessments included visual functions associated with lower and higher components of the cortical hierarchy, as well as dorsal- and ventral-related cortical functions. fMRI was performed on five patients to examine the neuronal substrate of their visual functions. The PCA patient cohort exhibited stereopsis, saccadic eye movements and higher dorsal stream-related functional impairments, including simultant perception, image orientation, figure-from-ground segregation, closure and spatial orientation. In accordance with the behavioral findings, fMRI revealed intact activation in the ventral visual regions of face and object perception while more dorsal aspects of perception, including motion and gestalt perception, revealed impaired patterns of activity. In most of the patients, there was a lack of activity in the word form area, which is known to be linked to reading disorders. Finally, there was evidence of reduced cortical representation of the peripheral visual field, corresponding to the behaviorally assessed peripheral visual deficit. The findings are discussed in the context of networks extending from parietal regions, which mediate navigationally related processing, visually guided actions, eye movement control and working memory, suggesting that damage to these networks might explain the wide range of deficits in PCA patients.







Similar content being viewed by others
References
Beh SC, Muthusamy B, Calabresi P et al (2015) Hiding in plain sight: a closer look at posterior cortical atrophy. Pract Neurol 15(1):5–13. doi:10.1136/practneurol-2014-000883
Benson D, Davis R, Snyder B (1988) Posterior cortical atrophy. Arch Neurol 7:193–203. doi:10.1684/pnv.2009.0169
Alves J, Soares JM, Sampaio A (2013) Posterior cortical atrophy and Alzheimer’s disease: a meta-analytic review of neuropsychological and brain morphometry studies. Brain Imaging Behav 7:353–361. doi:10.1007/s11682-013-9236-1
McMonagle P, Deering F, Berliner Y, Kertesz A (2006) The cognitive profile of posterior cortical atrophy. Neurology 66:331–338. doi:10.1212/01.wnl.0000196477.78548.db
Formaglio M, Costes N, Seguin J et al (2011) In vivo demonstration of amyloid burden in posterior cortical atrophy: a case series with PET and CSF findings. J Neurol 258:1841–1851. doi:10.1007/s00415-011-6030-0
Mendez MF, Ghajarania M, Perryman KM (2002) Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer’s disease. Dement Geriatr Cogn Disord 14:33–40. doi:10.1159/000058331
Kas A, de Souza LC, Samri D et al (2011) Neural correlates of cognitive impairment in posterior cortical atrophy. Brain 134:1464–1478. doi:10.1093/brain/awr055
Lehmann M, Crutch SJ, Ridgway GR et al (2011) Cortical thickness and voxel-based morphometry in posterior cortical atrophy and typical Alzheimer’s disease. NBA 32:1466–1476. doi:10.1016/j.neurobiolaging.2009.08.017
Garzia RP, Richman JE, Nicholson SB et al (1990) A new visual-verbal saccade test: the development eye movement test (DEM). J Am Optom Assoc 61:124–135
Katz J, Sommer A, Gaasterland DE et al (1991) Comparison of analytic algorithms for detecting glaucomatous visual field loss. Arch Ophthalmol 109:1684–1689. doi:10.1001/archopht.1991.01080120068028
Brown T, Elliott S (2011) Factor structure of the Motor-Free Visual Perception Test-3rd edition (MVPT-3). Can J Occup Ther 78:26–36. doi:10.2182/cjot.2011.78.1.4
Nicholas LE, Brookshire RH (1993) A system for quantifying the informativeness and efficiency of the connected speech of adults with aphasia. J Speech Lang Hear Res 36:338–350. doi:10.1044/jshr.3602.338
LaBarge E, Edwards D, Knesevich JW (1986) Performance of normal elderly on the Boston Naming Test. Brain Lang 27:380–384. doi:10.1016/0093-934X(86)90026-X
Hoffmann M, Keiseb J, Moodley J et al (2002) Appropriate neurological evaluation and multimodality magnetic resonance imaging in eclampsia. Acta Neurol Scand 106:159–167. doi:10.1034/j.1600-0404.2002.01255.x
Liberman J, Stewart W, Seines O et al (1994) Rater agreement for the Rey-Osterrieth Complex Figure Test. J Clin Psychol 50:615–624. doi:10.1002/1097-4679(199407)50:4<615:AID-JCLP2270500419>3.0.CO;2-R
Gollin ES (1960) Developmental studies of visual recognition of incomplete objects. Percept Mot Skills 11:289–298. doi:10.2466/pms.1960.11.3.289
Greve KW, Lindberg RF, Bianchini KJ et al (2000) Construct validity and predictive value of the Hooper Visual Organization Test in stroke rehabilitation. Appl Neuropsychol 7:215–222. doi:10.1016/0887-6177(94)00049-V
Della Sala S, Laiacona M, Trivelli C et al (1995) Poppelreuter-Ghent’s overlapping figures test: its sensitivity to age, and its clinical use. Arch Clin Neuropsychol 10:511–534. doi:10.1016/0887-6177(94)00049-V
Pillon B, Dubois B, Bonnet AM et al (1989) Cognitive slowing in Parkinson’s disease fails to respond to levodopa treatment: the 15-objects test. Neurology 39:762–768. doi:10.1212/WNL.39.6.762
Braddick O, Atkinson J, Wattam-Bell J (2003) Normal and anomalous development of visual motion processing: motion coherence and “dorsal-stream vulnerability”. Neuropsychologia 41:1769–1784. doi:10.1016/S0028-3932(03)00178-7
Rainville C, Marchand N, Passini R (2002) Performances of patients with a dementia of the Alzheimer type in the Standardized Road-Map test of Direction Sense. Neuropsychologia 40:567–573. doi:10.1016/S0028-3932(01)00133-6
Navon D (2003) What does a compound letter tell the psychologist’s mind? Acta Psychol (Amst) 114:273–309. doi:10.1016/j.actpsy.2003.06.002
Thomas C, Kveraga K, Huberle E et al (2012) Enabling global processing in simultanagnosia by psychophysical biasing of visual pathways. Brain 135:1578–1585. doi:10.1093/brain/aws066
Treisman A (1985) Preattentive processing in vision. Comput Vision Gr Image Process 31:156–177. doi:10.1016/S0734-189X(85)80004-9
Avidan G, Hasson U, Malach R et al (2005) Detailed exploration of face-related processing in congenital prosopagnosia: 2. Functional neuroimaging findings. J Cogn Neurosci 17:1150–1167. doi:10.1162/0898929054475145
Grill-Spector K, Kushnir T, Edelman S et al (1999) Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron 24:187–203. doi:10.1016/S0896-6273(00)80832-6
Cohen L, Lehéricy S, Chochon F et al (2002) Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain 125:1054–1069. doi:10.1093/brain/awf094
Tootell RB, Reppas JB, Kwong KK et al (1995) Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci 15:3215–3230
Engel SA, Glover GH, Wandell BA (1997) Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex 7:181–192. doi:10.1093/cercor/7.2.181
Boynton GM, Engel SA, Glover GH et al (1996) Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 16:4207–4221
Talairach J, Tournoux P (1988) Co-planar stereotaxis atlas of the human brain
Lee SY, Koo NK (2005) Change of stereoacuity with aging in normal eyes. Korean J Ophthalmol 19:136–139. doi:10.3341/kjo.2005.19.2.136
Sampedro A, Richman J, Pardo M (2003) The Adult Developmental Eye Movement Test (ADEM). J Behav Optom 14:101–105
Colarusso R, Hammill D (2003) MVPT-3: Motor-Free Visual Perception Test (MVPT)
Han AR, Kim DY, Choi TW et al (2014) Characteristics of visual-perceptual function measured by the motor-free visual perception test-3 in Korean adults. Ann Rehabil Med 38:548–553. doi:10.5535/arm.2014.38.4.548
Tamkin AS, Jacobsen R (1984) Age-related norms for the Hooper Visual Organization Test. J Clin Psychol 40:1459–1463. doi:10.1002/1097
Alegret M, Boada-Rovira M, Vinyes-Junqué G et al (2009) Detection of visuoperceptual deficits in preclinical and mild Alzheimer’s disease. J Clin Exp Neuropsychol 31:860–867. doi:10.1080/13803390802595568
Shin MS, Park SY, Park SR et al (2006) Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat Protoc 1:892–899. doi:10.1038/nprot.2006.115
Armstrong CL, Cloud B (1998) The emergence of spatial rotation deficits in dementia and normal aging. Neuropsychology 12:208–217. doi:10.1037/0894-4105.12.2.208
Brewer AA, Barton B (2014) Visual cortex in aging and Alzheimer’s disease: changes in visual field maps and population receptive fields. Front Psychol 5:74. doi:10.3389/fpsyg.2014.00074
Sauer J, Ffytche DH, Ballard C et al (2006) Differences between Alzheimer’s disease and dementia with Lewy bodies: an fMRI study of task-related brain activity. Brain 129:1780–1788. doi:10.1093/brain/awl102
Grill-Spector K, Knouf N, Kanwisher N (2004) The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci 7:555–562. doi:10.1038/nn1224
Huberle E, Karnath HO (2012) The role of temporo-parietal junction (TPJ) in global Gestalt perception. Brain Struct Funct 217:735–746. doi:10.1007/s00429-011-0369-y
Tang-Wai DF, Graff-Radford NR, Boeve BF et al (2004) Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology 63:1168–1174. doi:10.1212/01.WNL.0000140289.18472.15
Aresi A, Giovagnoli AR (2009) The role of neuropsychology in distinguishing the posterior cortical atrophy syndrome and Alzheimer’s disease. J Alzheimer Dis 18:65–70. doi:10.3233/JAD-2009-1123
Hochstein S, Ahissar M (2002) View from the top: hierarchies and reverse hierarchies in the visual system. Neuron 36:791–804. doi:10.1016/S0896-6273(02)01091-7
Schmidtke K, Talazko J, Hüll PKSM (2005) Posterior cortical atrophy: variant of Alzheimer’s disease? A case series with PET findings. J Neurol 252:27–35. doi:10.1007/s00415-005-0594-5
Andrade K, Kas A, Valabrègue R et al (2012) Visuospatial deficits in posterior cortical atrophy: structural and functional correlates. J Neurol Neurosurg Psychiatry 83:860–863. doi:10.1136/jnnp-2012-302278
Taylor J-P, Firbank MJ, He J et al (2012) Visual cortex in dementia with Lewy bodies: magnetic resonance imaging study. Br J Psychiatry 200:491–498. doi:10.1192/bjp.bp.111.099432
Yong KXX, Shakespeare TJ, Cash D et al (2014) (Con)text-specific effects of visual dysfunction on reading in posterior cortical atrophy. Cortex 57:92–106. doi:10.1016/j.cortex.2014.03.010
Cohen L, Martinaud O, Lemer C et al (2003) Visual word recognition in the left and right hemispheres: anatomical and functional correlates of peripheral alexias. Cereb Cortex 13:1313–1333. doi:10.1093/cercor/bhg079
Gaillard R, Naccache L, Pinel P et al (2006) Direct intracranial, fMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron 50:191–204. doi:10.1016/j.neuron.2006.03.031
Starrfelt R, Habekost T, Leff AP (2009) Too little, too late: reduced visual span and speed characterize pure alexia. Cereb Cortex 19:2880–2890. doi:10.1093/cercor/bhp059
Vogel AC, Miezin FM, Petersen SE, Schlaggar BL (2012) The putative visual word form area is functionally connected to the dorsal attention network. Cereb Cortex 22:537–549. doi:10.1093/cercor/bhr100
Pitzalis S, Galletti C, Huang R-S et al (2006) Wide-field retinotopy defines human cortical visual area v6. J Neurosci 26:7962–7973. doi:10.1523/JNEUROSCI.0178-06.2006
Stenbacka L, Vanni S (2007) fMRI of peripheral visual field representation. Clin Neurophysiol 118:1303–1314. doi:10.1016/j.clinph.2007.01.023
Kravitz DJ, Saleem KS, Baker CI et al (2011) A new neural framework for visuospatial processing. Nat Rev Neurosci 12:217–230. doi:10.1038/nrn3008
Conflicts of interest
The authors report no financial disclosure.
Ethical standards
The study was approved by the Hadassah Hebrew University Medical Center Ethics Committee and all patients signed a consent form allowing their participation in the study. The research has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shames, H., Raz, N. & Levin, N. Functional neural substrates of posterior cortical atrophy patients. J Neurol 262, 1751–1761 (2015). https://doi.org/10.1007/s00415-015-7774-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7774-8