Abstract
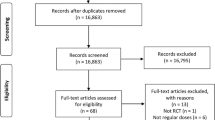
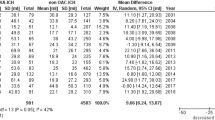
The new oral anticoagulants/non-vitamin K antagonists oral anticoagulants (NOACs) have recently reached the market and less is known about their safety in comparison to their efficacy. Therefore, we aimed to evaluate intracranial hemorrhage (ICH) risk with NOACs, the most feared adverse event of anticoagulation treatment. This is a systematic review and meta-analysis of phase III randomized controlled trials (RCTs) comparing NOACs versus any control and reporting ICH events. Studies were searched through Medline and Cochrane Library (April 2014). Reviews and reference lists were also screened. Random effects’ meta-analysis was performed to derive pooled estimates expressed as relative risk (RR) and 95 % CI. Number needed to treat/harm (NNT/NNH) taking into account the baseline risk was also calculated. Heterogeneity was evaluated with I 2 test. 18 RCTs evaluating 148,149 patients were included. NOAC significantly reduced ICH risk compared to vitamin K antagonists (VKA) (RR 0.44; 95 % CI 0.36–0.54; I 2 = 37 %; NNT: 137 during 2 years) and to sequential treatment with low molecular weight heparin and VKA (RR 0.28; 95 % CI 0.12–0.65; I 2 = 0 %; NNT: 463 patients during 7 months). Compared to placebo, NOACs were associated with an increased ICH risk (RR 3.31; 95 % CI 1.59–6.90; I 2 = 0 %; NNH: 433 during 1 year). Results were similar for the different NOAC drugs and across the different clinical conditions. In patients requiring anticoagulation treatment, the risk of ICH is about half with the NOACs in comparison to standard antithrombotic treatment. This safer profile found in RCTs should be confirmed in real-world database studies.

Similar content being viewed by others

References
Harder S, Graff J (2013) Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol 69:1617–1633
Landefeld CS, Beyth RJ (1993) Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 95:315–328
Linkins L, O’Donnell M, Julian JA, Kearon C (2010) Intracranial and fatal bleeding according to indication for long-term oral anticoagulant therapy. J Thromb Haemost 8:2201–2207
Fang MC, Go AS, Chang Y, Hylek EM, Henault LE, Jensvold NG et al (2007) Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 120:700–705
Bloom BJ, Filion KB, Atallah R, Eisenberg MJ (2014) Meta-analysis of randomized controlled trials on the risk of bleeding with dabigatran. Am J Cardiol 113:1066–1074
Wasserlauf G, Grandi SM, Filion KB, Eisenberg MJ (2013) Meta-analysis of rivaroxaban and bleeding risk. Am J Cardiol 112:454–460
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Lane PW (2013) Meta-analysis of incidence of rare events. Stat Methods Med Res 22:117–132
Turner RM, Bird SM, Higgins JP (2013) The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One 8:e59202
Kjaergard LL, Villumsen J, Gluud C (2001) Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 135:982–989
Zhang Z, Xu X, Ni H (2013) Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care 17:R2
Higgins JPT, Altman DG, Sterne JAC (2011). Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S (eds) Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration. http://www.cochrane-handbook.org
Deeks JJ (2002) Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med 21:1575–1600
Smeeth L, Haines A, Ebrahim S (1999) Numbers needed to treat derived from meta-analyses—sometimes informative, usually misleading. BMJ 318:1548–1551
DiCenso A (2001) Clinically useful measures of the effects of treatment. Evid Based Nurs. 4:36–39
Deeks JJ, Higgins JPT, Altman DG (2011) Chapter 9: analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (eds). Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration. http://www.cochrane-handbook.org
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2006) Comparison of two methods to detect publication bias in meta-analysis. JAMA 295:676–680
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ (2009) Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 361:594–604
Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P et al (2011) Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med 365:699–708
Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S et al (2011) Apixaban in patients with atrial fibrillation. N Engl J Med 364:806–817
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M et al (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365:981–992
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M et al (2013) Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 369:799–808
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M et al (2013) Apixaban for extended treatment of venous thromboembolism. N Engl J Med 368:699–708
Goldhaber SZ, Leizorovicz A, Kakkar AK, Haas SK, Merli G, Knabb RM et al (2011) Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med 365:2167–2177
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A et al (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361:1139–1151
Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H et al (2009) Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 361:2342–2352
Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D et al (2013) Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 368:709–718
Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, Hokusai-VTE Investigators et al (2013) Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 369:1406–1415
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL et al (2013) Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369:2093–2104
Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM et al (2009) Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 373:1673–1680
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W et al (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365:883–891
Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S et al (2012) Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation—the J-ROCKET AF study. Circ J 76(2104):11
Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C et al (2012) Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 366:9–19
Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, EINSTEIN–PE Investigators et al (2012) Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 366:1287–1297
Cohen AT, Spiro TE, Büller HR, Haskell L, Hu D, Hull R et al (2013) Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 368:513–523
Lovelock CE, Molyneux AJ, Rothwell PM, Oxford Vascular Study (2007) Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol 6:487–493
Thrift AG, McNeil JJ, Forbes A, Donnan GA (1999) Risk of primary intracerebral haemorrhage associated with aspirin and non-steroidal anti-inflammatory drugs: case–control study. BMJ 318:759–764
Linkins L, O’Donnell M, Julian JA, Kearon C (2010) Intracranial and fatal bleeding according to indication for long-term oral anticoagulant therapy. J Thromb Haemost 8:2201–2207
Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, Reilly PA et al (2012) Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke 43:1511–1517
Hankey GJ, Stevens SR, Piccini JP, Lokhnygina Y, Mahaffey KW, Halperin JL et al (2014) Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke 45:1304–1312
Deitelzweig S, Amin A, Jing Y, Makenbaeva D, Wiederkehr D, Lin J et al (2012) Medical cost reductions associated with the usage of novel oral anticoagulants vs warfarin among atrial fibrillation patients, based on the RE-LY, ROCKET-AF, and ARISTOTLE trials. J Med Econ 15:776–785
Canestaro WJ, Patrick AR, Avorn J, Ito K, Matlin OS, Brennan TA et al (2013) Cost-effectiveness of oral anticoagulants for treatment of atrial fibrillation. Circ Cardiovasc Qual Outcomes 6:724–731
Mahmoudi M, Sobieraj DM (2013) The cost-effectiveness of oral direct factor Xa inhibitors compared with low-molecular-weight heparin for the prevention of venous thromboembolism prophylaxis in total hip or knee replacement surgery. Pharmacotherapy 33:1333–1340
Acknowledgments
Portuguese Branch of the Iberoamerican Cochrane Centre. This was an academic project without any direct or indirect government or non-government funding.
Conflicts of interest
DC, MB and JC do not have any conflict of interests to disclose. JJF had speaker and consultant fees with GlaxoSmithKline, Novartis, TEVA, Lundbeck, Solvay, Abbott, Bial, Merck-Serono, Grunenthal, and Merck Sharp and Dohme. FJP had consultant and speaker fees with Astra Zeneca, Bayer and Boehringer Ingelheim.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Caldeira, D., Barra, M., Pinto, F.J. et al. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol 262, 516–522 (2015). https://doi.org/10.1007/s00415-014-7462-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7462-0