Abstract
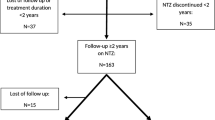
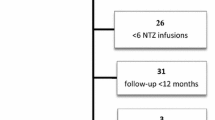
A number of studies have reported flare-up of multiple sclerosis (MS) disease activity after cessation of natalizumab, increasing to a level beyond the pre-natalizumab treatment level. Our aim was to describe the development in clinical disease activity following cessation of natalizumab therapy in a large unselected cohort of highly active patients. We studied 375 highly active patients who had suffered at least two significant relapses within 1 year or three relapses within 2 years, or had been treated with mitoxantrone for highly active disease. All patients had discontinued therapy with natalizumab after at least 24 weeks on therapy, and had been followed 3–12 months (mean 8.9 months) after cessation of natalizumab therapy. The annualised relapse rate before start of natalizumab therapy was 0.94 (95 % confidence interval [CI] 0.88–1.00), 0.47 (95 % CI 0.43–0.52) during natalizumab therapy, 0.63 (95 % CI 0.51–0.76) 1–6 months after natalizumab and 0.55 (95 % CI 0.42–0.70) 7–12 months after natalizumab. However, 83 (22 %) of the patients could be classified as showing rebound of relapses, defined as a higher individual relapse rate after cessation of natalizumab than before natalizumab. These patients had a higher annualised relapse rate during natalizumab therapy. For the whole patient group, the relapse rate after discontinuation did not exceed the pre-natalizumab relapse rate at any time, but 22 % of the patients showed rebound of relapses after discontinuation of natalizumab.


Similar content being viewed by others
References
O’Connor PW, Goodman A, Kappos L, Lublin FD, Miller DH, Polman C et al (2011) Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 76(22):1858–1865
Stuve O, Cravens PD, Frohman EM, Phillips JT, Remington GM, von Geldern G et al (2009) Immunologic, clinical, and radiologic status 14 months after cessation of natalizumab therapy. Neurology 72(5):396–401
West TW, Cree BA (2010) Natalizumab dosage suspension: are we helping or hurting? Ann Neurol 68(3):395–399
Kerbrat A, Le PE, Leray E, Anani T, Coustans M, Desormeaux C et al (2011) Natalizumab and drug holiday in clinical practice: an observational study in very active relapsing remitting multiple sclerosis patients. J Neurol Sci 308(1–2):98–102
Rigau V, Mania A, Befort P, Carlander B, Jonquet O, Lassmann H et al (2012) Lethal multiple sclerosis relapse after natalizumab withdrawal. Neurology 79(22):2214–2216
Rinaldi F, Seppi D, Calabrese M, Perini P, Gallo P (2012) Switching therapy from natalizumab to fingolimod in relapsing-remitting multiple sclerosis: clinical and magnetic resonance imaging findings. Mult Scler 18(11):1640–1643
Miravalle A, Jensen R, Kinkel RP (2011) Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol 68(2):186–191
Killestein J, Vennegoor A, Strijbis EM, Seewann A, van Oosten BW, Uitdehaag BM et al (2010) Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann Neurol 68(3):392–395
Papeix C, Depaz R, Tourbah A, Stankoff B, Lubetzki C (2011) Dramatic worsening following plasma exchange in severe post-natalizumab withdrawal multiple sclerosis relapse. Mult Scler 17(12):1520–1522
de Seze J, Ongagna JC, Collongues N, Zaenker C, Courtois S, Fleury M et al (2013) Reduction of the washout time between natalizumab and fingolimod. Mult Scler 19(9):1248
Laroni A, Brogi D, Milesi V, Abate L, Uccelli A, Mancardi G (2013) Early switch to fingolimod may decrease the risk of disease recurrence after natalizumab interruption. Mult Scler 19(9):1236–1237
Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA et al (2003) A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 348(1):15–23
Fox RJ, Cree BAC, De Sèze J, Gold R, Hartung H-P, Jeffery D, Kappos L, Kaufman M, Montalbán X, Weinstock-Guttman B, Anderson B, Natarajan A, Ticho B, Duda P. MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study, in press
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH et al (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354(9):899–910
Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW et al (2006) Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 354(9):911–923
Goodman AD, Rossman H, Bar-Or A, Miller A, Miller DH, Schmierer K et al (2009) GLANCE: results of a phase 2, randomized, double-blind, placebo-controlled study. Neurology 72(9):806–812
Hellwig K, Haghikia A, Gold R (2011) Pregnancy and natalizumab: results of an observational study in 35 accidental pregnancies during natalizumab treatment. Mult Scler 17(8):958–963
Rossi S, Motta C, Studer V, Monteleone F, De Chiara V, Buttari F et al (2013) A genetic variant of the anti-apoptotic protein Akt predicts natalizumab-induced lymphocytosis and post-natalizumab multiple sclerosis reactivation. Mult Scler 19(1):59–68
Hakiki B, Portaccio E, Giannini M, Razzolini L, Pasto L, Amato MP (2012) Withdrawal of fingolimod treatment for relapsing-remitting multiple sclerosis: report of six cases. Mult Scler 18(11):1636–1639
Havla JB, Pellkofer HL, Meinl I, Gerdes LA, Hohlfeld R, Kumpfel T (2012) Rebound of disease activity after withdrawal of fingolimod (FTY720) treatment. Arch Neurol 69(2):262–264
Borriello G, Prosperini L, Marinelli F, Fubelli F, Pozzilli C (2011) Observations during an elective interruption of natalizumab treatment: a post-marketing study. Mult Scler 17(3):372–375
Magraner MJ, Coret F, Navarre A, Bosca I, Simo M, Escutia M et al (2011) Pulsed steroids followed by glatiramer acetate to prevent inflammatory activity after cessation of natalizumab therapy: a prospective, 6-month observational study. J Neurol 258(10):1805–1811
Rossi S, Motta C, Studer V, De Chiara V, Barbieri F, Monteleone F et al (2013) Effect of glatiramer acetate on disease reactivation in MS patients discontinuing natalizumab. Eur J Neurol 20(1):87–94
Sempere AP, Martin-Medina P, Berenguer-Ruiz L, Perez-Carmona N, Sanchez-Perez R, Polache-Vengud J et al (2013) Switching from natalizumab to fingolimod: an observational study. Acta Neurol Scand 128(2):e6–e10
Havla J, Tackenberg B, Hellwig K, Meinl I, Krumbholz M, Seitz F et al (2013) Fingolimod reduces recurrence of disease activity after natalizumab withdrawal in multiple sclerosis. J Neurol 260(5):1382–1387
Conflicts of interest
Per Soelberg Sorensen received personal compensation from Biogen Idec, Merck Serono, Novartis, Genmab, TEVA, GSK, and Sanofi-aventis, Genzyme as member of scientific advisory boards, steering committees or independent data monitoring boards in clinical trials, or as a speaker at meetings. His research unit has received research support from Biogen Idec, Bayer Schering, Merck Serono, TEVA, Sanofi-aventis, Novartis, RoFAR, Roche, and Genzyme, the Danish Multiple Sclerosis Society, the Danish Medical Research Council, and the European Union Sixth Framework Programme: Life sciences, Genomics and Biotechnology for health. Nils Koch-Henriksen has received honoraria for lecturing and participation in advisory councils, travel expenses for attending congresses and meetings, and financial support for monitoring the Danish MS Treatment Register from Bayer, Merck-Serono, BiogenIdec, Sanofi-Avensis, Novartis, and TEVA. Thor Petersen has received funding or speaker honoraria from Biogen Idec, Merck Serono, Novartis, Bayer Schering, Sanofi-Aventis, Roche, and Genzyme. Mads Ravnborg has received travel grants and consultant honoraries from Bayer Health, Biogen-Idec, Sanofi Aventis, TEVA, Merck Serono, Genzyme and Novartis. Annette Oturai has served on scientific advisory boards for Novartis, and served as consultant for Biogen Idec; has received support for congress participation from Biogen Idec, Novartis, Sanofi Aventis and Teva; and has received speaker honoraria from Biogen Idec, Novartis, and Sanofi-Aventis. Her laboratory has received research support from Biogen Idec and Novartis. Finn Sellebjerg has served on scientific advisory boards, been on the steering committees of clinical trials, served as a consultant, received support for congress participation, received speaker honoraria, or received research support for his laboratory from Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novo Nordisk, Novartis, Sanofi-Aventis, Schering Plough and Teva.
Ethical standard
The study was approved by the Central Ethical Committee. All patients treated with disease-modifying drug are registered in the Danish Multiple Sclerosis Treatment Register as decided by the Danish National Board of Health. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sorensen, P.S., Koch-Henriksen, N., Petersen, T. et al. Recurrence or rebound of clinical relapses after discontinuation of natalizumab therapy in highly active MS patients. J Neurol 261, 1170–1177 (2014). https://doi.org/10.1007/s00415-014-7325-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7325-8