Abstract
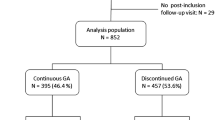
Five different immunomodulatory treatments have been developed and approved for the treatment of multiple sclerosis. These have been shown to be efficacious over the short-term in the reduction of relapse rates, measures of disease activity and disease progression in randomised, placebo-controlled trials. However, multiple sclerosis is a chronic progressive disease of variable course in which long-term benefits of treatment are critically important. It is not possible to obtain data on long-term outcome from placebo-controlled trials and observational open-label designs are required. Ideally, these would involve prospective follow-up of all included patients over decades. Long-term studies with β-interferons have generally used retrospective designs which have important limitations in terms of case ascertainment and selection bias. Nonetheless, they suggest that treatment benefit may be maintained over time. Glatiramer acetate is the only immunomodulatory treatment which has been studied prospectively over a period of ten years, and for which outcomes have been compared between subjects with continuous treatment and those in whom glatiramer acetate was discontinued. Ten-year data showed suppression of relapse rate to be maintained with 62% showing stable or improved disability levels. Disability progression was less than what would be expected from natural history studies of untreated patients with multiple sclerosis.
Similar content being viewed by others
References
Cohen JA, Cutter GR, Fischer JS, Goodman AD, Heidenreich FR, Kooijmans MF, Sandrock AW, Rudick RA, Simon JH, Simonian NA, Tsao EC, Whitaker JN (2002) IMPACT Investigators. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology 59:679–687
Confavreux C, Vukusic S, Moreau T, Adeleine P (2000) Relapses and progression of disability in multiple sclerosis. N Engl J Med 343:1430–1438
Ebers G, Traboulsee A, Langdon D, Goodin D, Konieczny A for the Betaseron LTF Study Group (2006) The interferon beta-1b 16-year long-term follow-up study: the results. Poster presented at AAN Annual Meeting, San Diego, CA, April 1–8, Poster number P01.079
European Study Group on interferon beta-1b in secondary progressive MS (1998). Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. Lancet 352:1491–1497
Ford CC, Johnson KP, Lisak RP, Panitch HS, Shifronis G, Wolinsky JS; Copaxone Study Group (2006) A prospective open-label study of glatiramer acetate: over a decade of continuous use in multiple sclerosis patients. Mult Scler 12:309–320
Jacobs LD, Cookfair DL, Rudick RA et al. (1996) Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 39:285–2
Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, Simonian NA, Slasor PJ, Sandrock AW (2000) Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 343:898–904
Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, Myers LW, Panitch HS, Rose JW, Schiffer RB (1995) Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 45:1268–1276
Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP et al. (1998) Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Neurology 50:701–708
Kinkel RP, Kollman C, O’Connor P, Murray TJ, Simon J, Arnold D, Bakshi R, Weinstock-Gutman B, Brod S, Cooper J, Duquette P, Eggenberger E, Felton W, Fox R, Freedman M, Galetta S, Goodman A, Guarnaccia J, Hashimoto S, Horowitz S, Javerbaum J, Kasper L, Kaufman M, Kerson L, Mass M, Rammohan K, Reiss M, Rolak L, Rose J, Scott T, Selhorst J, Shin R, Smith C, Stuart W, Thurston S, Wall M (2006) CHAMPIONS Study Group. IM interferon beta-1a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology 66:678–684
Kuhle J, Hardmeier M, D’hooghe MB, Schmierer K, Polman CH, Achiti C, Kappos L (2003) On behalf of the EUSPMS longterm follow-up (LTFU) Study Group. Longterm follow-up of the European Study of Interferon beta-1b (EUSPMS) in secondary progressive MS. Multiple Sclerosis 9 (Suppl 1):S143
Paty D, Kappos L, Moraga M, Stam Alsop J, Abdalla J for the PRISMS Study Investigators (2003) Long-term observational efficacy and safety follow-up of the PRISMS cohort. Poster presented at 19th ECTRIMS, Milan, Italy September 17–20:2003
Pittock SJ, Mayr WT, McClelland RL, Jorgensen NW, Weigand SD, Noseworthy JH, Weinshenker BG, Rodriguez M (2004) Change in MS-related disability in a population-based cohort: a 10-year follow-up study. Neurology 62:51–59
PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group (1998) Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 352:1498–1504
PRISMS Study Group and the University of British Columbia MS/MRI Analysis Group (2001) PRISMS-4: Long-term efficacy of interferon-beta-1a in relapsing MS. Neurology 56:1628–1636
SPECTRIMS (Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-beta-1a in MS) Study Group (2001) Randomized controlled trial of interferon-beta-1a in secondary progressive MS: clinical results. Neurology 56:1496–1504
The IFNB Multiple Sclerosis Study Group (1993) Interferon beta-1b is effective in relapsing–remitting multiple sclerosis. I. Clinical results of a multi-center, randomized, doubleblind, placebo-controlled trial. Neurology 43:655–661
The IFNB Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group (1995) Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 45:1277–1285
The North American Study Group on interferon beta-1b in Secondary Progressive MS (2004) Interferon beta-1b in secondary progressive MS: Results from a 3-year controlled study. Neurology 63:1788–1795
Weinshenker BG (1995) The natural history of multiple sclerosis. Neurol Clin 13:119–146
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ford, C.C. Long-term experience with current disease-modifying drugs in multiple sclerosis. J Neurol 253 (Suppl 6), vi37–vi44 (2006). https://doi.org/10.1007/s00415-006-6007-6
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-6007-6