Abstract
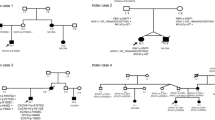
Epilepsy affects approximately 3 % of the world’s population, and sudden death is a significant cause of death in this population. Sudden unexpected death in epilepsy (SUDEP) accounts for up to 17 % of all these cases, which increases the rate of sudden death by 24-fold as compared to the general population. The underlying mechanisms are still not elucidated, but recent studies suggest the possibility that a common genetic channelopathy might contribute to both epilepsy and cardiac disease to increase the incidence of death via a lethal cardiac arrhythmia. We performed genetic testing in a large cohort of individuals with epilepsy and cardiac conduction disorders in order to identify genetic mutations that could play a role in the mechanism of sudden death. Putative pathogenic disease-causing mutations in genes encoding cardiac ion channel were detected in 24 % of unrelated individuals with epilepsy. Segregation analysis through genetic screening of the available family members and functional studies are crucial tasks to understand and to prove the possible pathogenicity of the variant, but in our cohort, only two families were available. Despite further research should be performed to clarify the mechanism of coexistence of both clinical conditions, genetic analysis, applied also in post-mortem setting, could be very useful to identify genetic factors that predispose epileptic patients to sudden death, helping to prevent sudden death in patients with epilepsy.

Similar content being viewed by others
References
Annegers JF (2001) The epidemiology of epilepsy. In: Wyllie E (ed) The treatment of epilepsy: principles and practice, 3rd edn. Lippincott Williams & Wilkins, Philadelphia, pp 131–138
Shorvon S, Tomson T (2011) Sudden unexpected death in epilepsy. Lancet 378(9808):2028–38
Hughes JR (2009) A review of sudden unexpected death in epilepsy: prediction of patients at risk. Epilepsy Behav 14:280–287
Lhatoo SD, Johnson AL, Goodridge DM et al (2001) Mortality in epilepsy in the first 11 to 14 years after diagnosis: multivariate analysis of a long-term, prospective, population-based cohort. Ann Neurol 49:336–344
Walczak TS, Leppik IE, D’Amelio M et al (2001) Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology 56:519–525
Johnston A, Smith P (2007) Sudden unexpected death in epilepsy. Expert Rev Neurother 7:1751–1761
Lee BI, Heo K (2014) Epilepsy: new genes, new technologies, new insights. Lancet Neurol 13(1):7–9
Nashef L et al (2007) Risk factors in sudden death in epilepsy (SUDEP): the quest for mechanisms. Epilepsia 48(5):859–871
Jansen K, Lagae L (2010) Cardiac changes in epilepsy. Seizure 19:455–460
Kim JB (2014) Channelopathies. Korean J Pediatr 57(1):1–18
Nashef L, Hindocha N, Makoff A (2007) Risk factors in sudden death in epilepsy (SUDEP): the quest for mechanisms. Epilepsia 48:859–871
Glasscock E, Yoo JW, Chen TT et al (2010) Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci 30(15):5167–75
Haugaa KH, Vestervik TT, Andersson S, Amlie JP, Jørum E, Gjerstad L, Taubøll E. Abnormal electroencephalograms in patients with long QT syndrome. Heart Rhythm. 2013 Sep 27. pii: S1547-5271(13)01081-3
Sandorfi G, Clemens B, Csanadi Z (2013) Electrical storm in the brain and in the heart: epilepsy and Brugada syndrome. Mayo Clin Proc 88(10):1167–1173
Jeppesen J, Fuglsang-Frederiksen A, Brugada R, Pedersen B, Rubboli G, Johansen P, Beniczky S (2014) Heart rate variability analysis indicates preictal parasympathetic overdrive preceding seizure-induced cardiac dysrhythmias leading to sudden unexpected death in a patient with epilepsy. Epilepsia
Klassen TL, Bomben VC, Patel A et al (2014) High-resolution molecular genomic autopsy reveals complex sudden unexpected death in epilepsy risk profile. Epilepsia 55(2):e6–12
Kersey PJ et al (2012) Ensembl Genomes: an integrative resource for genome-scale data from non-vertebrate species. Nucleic Acids Res 40:D91
A map of human genome variation from population-scale sequencing. Nature 467, 1061 (Oct 28, 2010)
Stenson PD et al (2003) Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 21:577
S. T. Sherry et al., dbSNP: the NCBI database of genetic variation. Nucleic acids research 29, 308 (Jan 1, 2001).
Gonzalez-Perez A, Lopez-Bigas N (2011) Improving the assessment of the outcome of nonsynonymous SNVs with a consensus deleteriousness score, Condel. Am J Hum Genet 88:440
Schwarz JM, Rödelsperger C, Schuelke M et al (2010) MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 7(8):575–6
den Dunnen JT, Antonarakis SE (2001) Nomenclature for the description of human sequence variations. Hum Genet 109(1):121–4
Surges R, Thijs RD, Tan HL (2009) Sander JW Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol 5:492–504
Campuzano O, Beltrán-Alvarez P, Iglesias A et al (2010) Genetics and cardiac channelopathies. Genet Med 12(5):260–7
Heron SE, Hernandez M, Edwards C et al (2010) Neonatal seizures and long QT syndrome: a cardiocerebral channelopathy. Epilepsia 51:293–296
Johnson JN, Hofman N, Haglund CM et al (2009) Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology 72:224–231
Catalano O, Antonaci S, Moro G et al (2009) Magnetic resonance investigations in Brugada syndrome reveal unexpectedly high rate of structural abnormalities. Eur Heart J 30(18):2241–2248
Tu E, Bagnall RD, Duflou J et al (2011) Post-mortem review and genetic analysis of sudden unexpected death in epilepsy (SUDEP) cases. Brain Pathol 21:201–208
Anderson JH, Bos JM, Meyer FB et al (2012) Concealed long QT syndrome and intractable partial epilepsy: a case report. Mayo Clin Proc 87:1128–1131
Partemi S, Cestèle S, Pezzella M et al (2013) Loss-of-function KCNH2 mutation in a family with long QT syndrome, epilepsy, and sudden death. Epilepsia 54(8):e112–6
Zamorano-León JJ, Yañez R, Jaime G et al (2012) KCNH2 gene mutation: a potential link between epilepsy and long QT-2 syndrome. J Neurogenet 26(3–4):382–6
Parisi P, Oliva A, Coll Vidal M et al (2013) Coexistence of epilepsy and Brugada syndrome in a family with SCN5A mutation. Epilepsy Res 105(3):415–8
Campuzano O, Berne P, Selga E, Allegue C, Iglesias A, Brugada J, Brugada R, Brugada syndrome and p.E61X_ RANGRF. Cardiol J. 2014 Mar 27
Omichi C, Momose Y, Kitahara S (2010) Congenital long QT syndrome presenting with a history of epilepsy: misdiagnosis or relationship between channelopathies of the heart and brain? Epilepsia 51(2):289–92
Arbustini E, Scaffino MF, Diegoli M et al (2005) Gene symbol: SCN5A. Disease: Brugada syndrome. Hum Genet 118(3-4):536
Risgaard B, Jabbari R, Refsgaard L et al (2013) High prevalence of genetic variants previously associated with Brugada syndrome in new exome data. Clin Genet 84(5):489–95
Vatta M, Dumaine R, Varghese G et al (2002) Genetic and biophysical basis of sudden unexplained nocturnal death syndrome (SUNDS), a disease allelic to Brugada syndrome. Hum Mol Genet 11(3):337–45
Nakajima T, Kaneko Y, Saito A et al (2011) Identification of six novel SCN5A mutations in Japanese patients with Brugada syndrome. Int Heart J 52(1):27–31
Wang Q, Chen S, Chen Q et al (2004) The common SCN5A mutation R1193Q causes LQTS-type electrophysiological alterations of the cardiac sodium channel. J Med Genet 41(5):e66
Sun A, Xu L, Wang S et al (2008) SCN5A R1193Q polymorphism associated with progressive cardiac conduction defects and long QT syndrome in a Chinese family. J Med Genet 45:127–128
Hwang HW, Chen JJ, Lin YJ et al (2005) R1193Q of SCN5A, a Brugada and long QT mutation, is a common polymorphism in Han Chinese. J Med Genet 42(2):e7
Refsgaard L, Holst AG, Sadjadieh G et al (2012) High prevalence of genetic variants previously associated with LQT syndrome in new exome data. Eur J Hum Genet 20(8):905–8
Wang Q, Chen S, Chen Q, Wan X, Shen J, Hoeltge GA, Timur AA, et al. The common SCN5A mutation R1193Q causes LQTS-type electrophysiological alterations of the cardiac sodium channel. J Med Genet 2004
Tan BH, Valdivia CR, Rok BA et al (2005) Common human SCN5A polymorphisms have altered electrophysiology when expressed in Q1077 splice variants. Heart Rhythm 2(7):741–7
Ackerman MJ, Splawski I, Makielski JC et al (2004) Spectrum and prevalence of cardiac sodium channel variants among black, white, Asian, and Hispanic individuals: implications for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm 1(5):600–7
Nishio H, Kuwahara M, Tsubone H et al (2009) Identification of an ethnic-specific variant (V207M) of the KCNQ1 cardiac potassium channel gene in sudden unexplained death and implications from a knock-in mouse model. Int J Legal Med 123(3):253–7
Giudicessi JR, Kapplinger JD, Tester DJ et al (2012) Phylogenetic and physicochemical analyses enhance the classification of rare non-synonymous single nucleotide variants in type 1 and 2 long-QT syndrome. Circ Cardiovasc Genet 5:519–528
Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Ackerman MJ, Tester DJ, Jones GS, Will ML, Burrow CR, Curran ME. Mayo Clin Proc. 2003 Dec;78(12):1479-87
Yang T, Chung SK, Zhang W et al (2009) Biophysical properties of 9 KCNQ1 mutations associated with long-QT syndrome. Circ Arrhythm Electrophysiol 2(4):417–26
Khositseth A, Tester DJ, Will ML et al (2004) Identification of a common genetic substrate underlying postpartum cardiac events in congenital long QT syndrome. Heart Rhythm 1(1):60–4
Nishio Y, Makiyama T, Itoh H et al (2009) D85N, a KCNE1 polymorphism, is a disease-causing gene variant in long QT syndrome. J Am Coll Cardiol 54(9):812–9
Lieve KV, Williams L, Daly A et al (2013) Results of genetic testing in 855 consecutive unrelated patients referred for long QT syndrome in a clinical laboratory. Genet Test Mol Biomark 17(7):553–61
Yoshikane Y, Yoshinaga M, Hamamoto K et al (2013) A case of long QT syndrome with triple gene abnormalities: digenic mutations in KCNH2 and SCN5A and gene variant in KCNE1. Heart Rhythm 10(4):600–3
Madea B, Saukko P, Oliva A et al (2010) Molecular pathology in forensic medicine—introduction. Forensic Sci Int 203(1–3):3–14
Partemi S, Berne PM, Batlle M et al (2010) Analysis of mRNA from human heart tissue and putative applications in forensic molecular pathology. Forensic Sci Int 203(1–3):99–105
Acknowledgments
This work was supported by the Cardiovascular Genetics Center, University of Girona, Spain, and by Telethon Foundation Italy, Grant GGP10186A. We wish also to thank the Collaborative Group on SUDEP of Italian League Against Epilepsy (LICE) for the support in sample collections.
Authors’ contribution
The study concept or design was made by SP, MCV, PS, AO, and RB. All authors draft/revise the manuscript. All the authors made the analysis of interpretation.
Conflict of interests
The authors declare that they have no competing interests.
Disclosure
All authors report no disclosure.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sara Partemi and Monica Coll Vidal equally contributed to this study.
Antonio Oliva and Ramon Brugada are co-senior authors
Rights and permissions
About this article
Cite this article
Partemi, S., Vidal, M.C., Striano, P. et al. Genetic and forensic implications in epilepsy and cardiac arrhythmias: a case series. Int J Legal Med 129, 495–504 (2015). https://doi.org/10.1007/s00414-014-1063-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-014-1063-4