Abstract
Background
Lung cancer is predominantly a disease of the elderly: half of all newly diagnosed patients are over 70 years old. Older patients and those with comorbidities are underrepresented in clinical trials; scientific communities have addressed this issue since the end of the 20th century. We set out to determine the characteristics of the selection of patients in lung cancer trials that are currently recruiting.
Methods
We searched The United States National Institutes of Health (NIH) clinical trial registry (www.clinicaltrials.gov) on April 23, 2015 for currently recruiting phase I, II, or III clinical trials in lung cancer. Trial characteristics and study objectives were extracted from the registry website.
Results
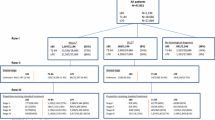
Of the 419 trails selected in this overview, 88 % explicitly or implicitly excluded elderly patients. Patients were excluded based on stringent organ selection in 76 % of the trials, based on performance status (57 %) and based on age (13 %). The median number of placed restrictions per trial was seven. In the 2 % of the trials that were exclusively designed for elderly patients only fit patients were included.
Conclusion
In this overview of current lung cancer trials registered in the NIH clinical trial registry, we found that elderly patients and those with comorbidities are often excluded from participation in clinical trials. Therefore, it is difficult for physicians and their frail patients to properly evaluate the efficacy and safety of current treatment options. More research that includes the elderly and those with comorbidities is urgently needed.
Similar content being viewed by others
Abbreviations
- NIH:
-
National Institutes of Health
- NSCLC:
-
Non-small cell lung cancer
- PS:
-
Performance status
- SCLC:
-
Small cell lung cancer
- WHO:
-
World Health Organization
References
Longkanker (2016) http://www.cijfersoverkanker.nl. Accessed 1st Jan 2016
Kastelijn EA, El Sharouni SY, Hofman FN, Van Putte BP, Monninkhof EM, Van Vulpen M, Schramel F (2015) Clinical outcomes in early-stage NSCLC treated with stereotactic body radiotherapy versus surgical resection. Anticancer Res 35:5607–5614
Gridelli C et al (2010) Recent issues in first-line treatment of advanced non-small-cell lung cancer: results of an international expert panel meeting of the italian association of thoracic oncology. Lung Cancer 68:319–331
Chen Y-M et al (2012) Phase II randomized trial of erlotinib or vinorelbine in chemonaive, advanced, non-small cell lung cancer patients aged 70 years or older. J Thorac Oncol 7:412–418
Hutchins LF, Unger JM, Crowley J et al (1999) Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 34:2061–2067
Murthy VH, Krumholz HM, Gross CP (2004) Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 291:2720–2726
Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S et al (2005) Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the international society of geriatric oncology (SIOG). Crit Rev Oncol Hematol 55:241–252
Hamaker ME, Prins MC (2014) S. R. The relevance of geriatric assessment for elderly patients with a haematological malignancy—A systematic review. Leuk Res. doi:10.1016/j.leukres.2013.12.018
Hurria A, Mohile SG, Dale W (2012) Research priorities in geriatric oncology: addressing the needs of an aging population. J Natl Compr Cancer Netw 10:286–288
Pallis AG, Ring A, Fortpied C et al (2011) EORTC workshop on clinical trial methodology in older individuals with a diagnosis of solid tumors. Ann Oncol 22:1922–1926
Hurria A, Cohen HJ, Extermann M (2010) Geriatric oncology research in the cooperative groups: a report of a SIOG special meeting. J. Geriatr Oncol 1:40–44
Jennens RR, Giles GG, Fox RM (2006) Increasing underrepresentation of elderly patients with advanced colorectal or non-small-cell lung cancer in chemotherapy trials. Intern Med J 36:216–220
Lewis JH et al (2003) Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 21:1383–1389
Ma C, Bandukwala S, Burman D et al (2010) Interconversion of three measures of performance status: an empirical analysis. Eur J Cancer 46:3175–3183
Hamaker ME, Stauder R, van Munster BC (2014) Exclusion of older patients from ongoing clinical trials for hematological malignancies: an evaluation of the National institutes of health clinical trial registry. Oncologist 19:1069–1075
Wildiers H et al (2013) End points and trial design in geriatric oncology research: a joint European organisation for research and treatment of cancer–Alliance for clinical trials in oncology-international society of geriatric oncology position article. J Clin Oncol 31:3711–3718
Mol L, Koopman M, van Gils C, Ottevanger P, Punt C (2013) Comparison of treatment outcome in metastatic colorectal cancer patients included in a clinical trial versus daily practice in The Netherlands. Acta Oncol 52:950–955
The elderly lung cancer vinorelbine italian study group (1999) Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 91:66–72
Beretta GD et al (2000) Gemcitabine plus vinorelbine in elderly or unfit patients with non-small cell lung cancer. Br J Cancer 83:573–576
Earle CC et al (2001) Effectiveness of chemotherapy for advanced lung cancer in the elderly: instrumental variable and propensity analysis. J Clin Oncol 19:1064–1070
Weinmann M, Jeremic B, Toomes H, Friedel G, Bamberg M (2003) Treatment of lung cancer in the elderly. Part I: non-small cell lung cancer. Lung Cancer 39:233–253
Chen H et al (2003) Can older cancer patients tolerate chemotherapy? Cancer 97:1107–1114
Rocha Lima CMS, Herndon JE, Kosty M, Clamon G, Green MR (2002) Therapy choices among older patients with lung carcinoma: an evaluation of two trials of the cancer and Leukemia group B. Cancer 94:181–187
Magne N et al (2002) Palliative 5-fluorouracil-based chemotherapy for advanced colorectal cancer in the elderly: results of a 10-year experience. Am J Clin Oncol 25:126–130
Hennessy BT, Hanrahan EO, Breathnach OS (2003) Chemotherapy options for the elderly patient with advanced non-small cell lung cancer. Oncologist 8:270–277
Yee KWL, Pater JL, Pho L, Zee B, Siu LL (2003) Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol 21:1618–1623
Acknowledgment
This study was supported by the Aart Huisman Scholarship for research in geriatric oncology and the Cornelis Visser Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest.
Appendix
Rights and permissions
About this article
Cite this article
Schulkes, K.J., Nguyen, C., van den Bos, F. et al. Selection of Patients in Ongoing Clinical Trials on Lung Cancer. Lung 194, 967–974 (2016). https://doi.org/10.1007/s00408-016-9943-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-016-9943-7