Abstract
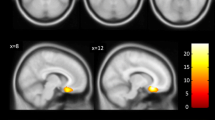
The diacylglycerol kinase eta (DGKH) gene, first identified in a genome-wide association study, is one of the few replicated risk genes of bipolar affective disorder (BD). Following initial positive studies, it not only was found to be associated with BD but also implicated in the etiology of other psychiatric disorders featuring affective symptoms, rendering DGKH a cross-disorder risk gene. However, the (patho-)physiological role of the encoded enzyme is still elusive. In the present study, we investigated primarily the influence of a risk haplotype on amygdala volume in patients suffering from schizophrenia or BD as well as healthy controls and four single nucleotide polymorphisms conveying risk. There was a significant association of the DGKH risk haplotype with increased amygdala volume in BD, but not in schizophrenia or healthy controls. These findings add to the notion of a role of DGKH in the pathogenesis of BD.


Similar content being viewed by others
References
Abrial E, Lucas G, Scarna H, Haddjeri N, Lambas-Senas L (2011) A role for the PKC signaling system in the pathophysiology and treatment of mood disorders: involvement of a functional imbalance? Mol Neurobiol 44:407–419
Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM (2009) Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry 195:194–201
Barnett JH, Smoller JW (2009) The genetics of bipolar disorder. Neuroscience 164:331–343
Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, Schulze TG, Cichon S, Rietschel M, Nothen MM, Georgi A, Schumacher J, Schwarz M, Abou Jamra R, Hofels S, Propping P, Satagopan J, Detera-Wadleigh SD, Hardy J, McMahon FJ (2008) A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry 13:197–207
Blond BN, Fredericks CA, Blumberg HP (2012) Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala-anterior paralimbic neural system. Bipolar Disord 14:340–355
Bone R, Springer JP, Atack JR (1992) Structure of inositol monophosphatase, the putative target of lithium therapy. Proc Natl Acad Sci USA 89:10031–10035
Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN (2013) Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. doi:10.1093/cercor/bht154
Chen G, Masana MI, Manji HK (2000) Lithium regulates PKC-mediated intracellular cross-talk and gene expression in the CNS in vivo. Bipolar Disord 2:217–236
Cross-Disorder Group of the Psychiatric Genomics Consortium; Genetic Risk Outcome of Psychosis (GROUP) Consortium (2013) Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379
DelBello MP, Adler CM, Strakowski SM (2006) The neurophysiology of childhood and adolescent bipolar disorder. CNS Spectr 11:298–311
Domschke K, Reif A (2012) Behavioral genetics of affective and anxiety disorders. Curr Top Behav Neurosci 12:463–502
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Foland LC, Altshuler LL, Sugar CA, Lee AD, Leow AD, Townsend J, Narr KL, Asuncion DM, Toga AW, Thompson PM (2008) Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport 19:221–224
Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S (2001) Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology 158:100–106
Hajek T, Cullis J, Novak T, Kopecek M, Hoschl C, Blagdon R, O’Donovan C, Bauer M, Young LT, Macqueen G, Alda M (2012) Hippocampal volumes in bipolar disorders: opposing effects of illness burden and lithium treatment. Bipolar Disord 14:261–270
Hajek T, Kopecek M, Hoschl C, Alda M (2012) Smaller hippocampal volumes in patients with bipolar disorder are masked by exposure to lithium: a meta-analysis. J Psychiatry Neurosci 37:333–343
Hajek T, Kopecek M, Kozeny J, Gunde E, Alda M, Hoschl C (2009) Amygdala volumes in mood disorders—meta-analysis of magnetic resonance volumetry studies. J Affect Disord 115:395–410
Hallahan B, Newell J, Soares JC, Brambilla P, Strakowski SM, Fleck DE, Kieseppa T, Altshuler LL, Fornito A, Malhi GS, McIntosh AM, Yurgelun-Todd DA, Labar KS, Sharma V, MacQueen GM, Murray RM, McDonald C (2011) Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biol Psychiatry 69:326–335
Honea R, Crow TJ, Passingham D, Mackay CE (2005) Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 162:2233–2245
Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM (2009) Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 373:234–239
Manji HK, Lenox RH (1999) Ziskind-Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol Psychiatry 46:1328–1351
Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK (2000) Lithium-induced increase in human brain grey matter. Lancet 356:1241–1242
Moya PR, Murphy DL, McMahon FJ, Wendland JR (2010) Increased gene expression of diacylglycerol kinase eta in bipolar disorder. Int J Neuropsychopharmacol 13:1127–1128
Ollila HM, Soronen P, Silander K, Palo OM, Kieseppa T, Kaunisto MA, Lonnqvist J, Peltonen L, Partonen T, Paunio T (2009) Findings from bipolar disorder genome-wide association studies replicate in a Finnish bipolar family-cohort. Mol Psychiatry 14:351–353
Pessoa L, Adolphs R (2010) Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci 11:773–783
Pfeifer JC, Welge J, Strakowski SM, Adler CM, DelBello MP (2008) Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 47:1289–1298
Pfennig A, Bschor T, Falkai P, Bauer M (2013) The diagnosis and treatment of bipolar disorder: recommendations from the current s3 guideline. Dtsch Arztebl Int 110:92–100
Sakane F, Kanoh H (1997) Molecules in focus: diacylglycerol kinase. Int J Biochem Cell Biol 29:1139–1143
Savitz J, Frank MB, Victor T, Bebak M, Marino JH, Bellgowan PS, McKinney BA, Bodurka J, Kent Teague T, Drevets WC (2012) Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain Behav Immun 31:161–171. doi:10.1016/j.bbi.2012.10.007
Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL, Gattaz WF, Bogerts B, Schmitz C, Falkai P (2009) Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol 117(4):395–407. doi:10.1007/s00401-008-0430-y
Smoller JW, Craddock N, Kendler K, Lee PH, Neale BM, Nurnberger JI, Ripke S, Santangelo S, Sullivan PF (2013) Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379
Stambolic V, Ruel L, Woodgett JR (1996) Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol 6:1664–1668
Steckert AV, Valvassori SS, Mina F, Lopes-Borges J, Varela RB, Kapczinski F, Dal-Pizzol F, Quevedo J (2012) Protein kinase C and oxidative stress in an animal model of mania. Curr Neurovasc Res 9:47–57
Swayze VW 2nd, Andreasen NC, Alliger RJ, Ehrhardt JC, Yuh WT (1990) Structural brain abnormalities in bipolar affective disorder. Ventricular enlargement and focal signal hyperintensities. Arch Gen Psychiatry 47:1054–1059
Swayze VW 2nd, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC (1992) Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 31:221–240
Takata A, Kawasaki H, Iwayama Y, Yamada K, Gotoh L, Mitsuyasu H, Miura T, Kato T, Yoshikawa T, Kanba S (2011) Nominal association between a polymorphism in DGKH and bipolar disorder detected in a meta-analysis of East Asian case-control samples. Psychiatry Clin Neurosci 65:280–285
Tesli M, Kahler AK, Andreassen BK, Werge T, Mors O, Mellerup E, Koefoed P, Melle I, Morken G, Wirgenes KV, Andreassen OA, Djurovic S (2009) No association between DGKH and bipolar disorder in a Scandinavian case-control sample. Psychiatr Genet 19:269–272
Topham MK, Epand RM (2009) Mammalian diacylglycerol kinases: molecular interactions and biological functions of selected isoforms. Biochim Biophys Acta 1790:416–424
Townsend J, Altshuler LL (2012) Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord 14:326–339
Tu-Sekine B, Goldschmidt H, Petro E, Raben DM (2013) Diacylglycerol kinase theta: regulation and stability. Adv Biol Regul 53:118–126
Usher J, Leucht S, Falkai P, Scherk H (2010) Correlation between amygdala volume and age in bipolar disorder—a systematic review and meta-analysis of structural MRI studies. Psychiatry Res 182:1–8
Usher J, Menzel P, Schneider-Axmann T, Kemmer C, Reith W, Falkai P, Gruber O, Scherk H (2010) Increased right amygdala volume in lithium-treated patients with bipolar I disorder. Acta Psychiatr Scand 121:119–124
van Dongen J, Boomsma DI (2013) The evolutionary paradox and the missing heritability of schizophrenia. Am J Med Genet B Neuropsychiatr Genet 162B:122–136
Weber H, Kittel-Schneider S, Gessner A, Domschke K, Neuner M, Jacob CP, Buttenschon HN, Boreatti-Hummer A, Volkert J, Herterich S, Baune BT, Gross-Lesch S, Kopf J, Kreiker S, Nguyen TT, Weissflog L, Arolt V, Mors O, Deckert J, Lesch KP, Reif A (2011) Cross-disorder analysis of bipolar risk genes: further evidence of DGKH as a risk gene for bipolar disorder, but also unipolar depression and adult ADHD. Neuropsychopharmacology 36(10):2076–2085. doi:10.1038/npp.2011.98
Whalley HC, Papmeyer M, Romaniuk L, Johnstone EC, Hall J, Lawrie SM, Sussmann JE, McIntosh AM (2012) Effect of variation in diacylglycerol kinase eta (DGKH) gene on brain function in a cohort at familial risk of bipolar disorder. Neuropsychopharmacology 37:919–928
Wijeratne C, Sachdev S, Wen W, Piguet O, Lipnicki DM, Malhi GS, Mitchell PB, Sachdev PS (2013) Hippocampal and amygdala volumes in an older bipolar disorder sample. Int Psychogeriatr 25:54–60
Wolf C, Mohr H, Schneider-Axmann T, Reif A, Wobrock T, Scherk H, Kraft S, Schmitt A, Falkai P, Gruber O (2013) CACNA1C genotype explains interindividual differences in amygdala volume among patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 264(2):93–102. doi:10.1007/s00406-013-0427-y
Yasuda S, Kai M, Imai S, Takeishi K, Taketomi A, Toyota M, Kanoh H, Sakane F (2009) Diacylglycerol kinase eta augments C-Raf activity and B-Raf/C-Raf heterodimerization. J Biol Chem 284:29559–29570
Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M, O’Donovan C, Macqueen G, McIntyre RS, Sharma V, Ravindran A, Young LT, Milev R, Bond DJ, Frey BN, Goldstein BI, Lafer B, Birmaher B, Ha K, Nolen WA, Berk M (2013) Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 15:1–44
Yosifova A, Mushiroda T, Kubo M, Takahashi A, Kamatani Y, Kamatani N, Stoianov D, Vazharova R, Karachanak S, Zaharieva I, Dimova I, Hadjidekova S, Milanova V, Madjirova N, Gerdjikov I, Tolev T, Poryazova N, O’Donovan MC, Owen MJ, Kirov G, Toncheva D, Nakamura Y (2011) Genome-wide association study on bipolar disorder in the Bulgarian population. Genes Brain Behav 10:789–797
Yu K, Cheung C, Leung M, Li Q, Chua S, McAlonan G (2010) Are bipolar disorder and schizophrenia neuroanatomically distinct? An anatomical likelihood meta-analysis. Front Hum Neurosci 4:189
Zeng Z, Wang T, Li T, Li Y, Chen P, Zhao Q, Liu J, Li J, Feng G, He L, Shi Y (2011) Common SNPs and haplotypes in DGKH are associated with bipolar disorder and schizophrenia in the Chinese Han population. Mol Psychiatry 16:473–475
Acknowledgments
This study was supported by the DFG (Grant RTG 1253/1, RE1632/5-1 to AR), the BMBF (DZHI, Project 01EO1004, to AR), and the IZKF (Project Z3-24, to SKS). I. Reck and C. Gagel are credited for excellent technical assistance.
Conflict of interest
S. Kittel-Schneider, B. Malchow, H. Scherk, C. Wolf, S. Trost, M. Backens, D. Zilles, W. Reith, and T. Schneider-Axmann declare no conflicts of interest. A. Hasan has been invited to scientific conferences by Janssen-Cilag, Pfizer, and Lundbeck. He received paid speakership by Desitin. O. Gruber was an honorary speaker for AstraZeneca, Bristol-Meyers Squibb, Janssen-Cilag, and Otsuka and has been invited for scientific congresses by AstraZeneca, Janssen-Cilag, and Pfizer. A. Schmitt was honorary speaker for TAD Pharma and Roche and has been member of advisory boards for Roche. P. Falkai has been member of the advisory boards for Janssen-Cilag, BMS, Lundback, Pfizer, Lilly, and AstraZeneca and received an educational grant from AstraZeneca and honoraria as lecturer from Janssen-Cilag, BMS, Lundbeck, Pfizer, Lilly, and AstraZeneca. T. Wobrock received honoraria and has participated in speaker bureaus for Alpine Biomed, AstraZeneca, Bristol-Myers Squibb, Lilly, Essex, Organon, Janssen-Cilag, Pfizer, Sanofi-Synthelabo/Aventis and Lundbeck and a research grant from AstraZeneca. A. Reif has received research grants from Astra Zeneca.
Author information
Authors and Affiliations
Corresponding author
Additional information
O. Gruber and A. Reif have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kittel-Schneider, S., Wobrock, T., Scherk, H. et al. Influence of DGKH variants on amygdala volume in patients with bipolar affective disorder and schizophrenia. Eur Arch Psychiatry Clin Neurosci 265, 127–136 (2015). https://doi.org/10.1007/s00406-014-0513-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-014-0513-9