Abstract
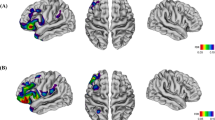
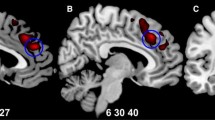
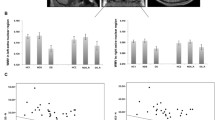
The neurobiological correlates of impaired insight in psychotic illness remain uncertain and may be confounded by factors such as illness progression and medication use. Our study consisted of two separate experiments. In the first experiment, we examined the association between measures of insight and regional brain volume in thirty-two patients with first-episode psychosis. In the second experiment, we looked at similar associations in thirty individuals with chronic schizophrenia. Detailed measures of symptom awareness and symptom attribution were obtained using the Scale to assess Unawareness of Mental Disorder. MRI scans were acquired and analysed using Statistical Non-Parametric Mapping for voxel-based analyses of grey matter maps. Regression models were used to assess the relationship between insight and grey matter volume in both the first-episode psychosis and the chronic schizophrenia experiments whilst controlling for potential confounds. In first-episode psychosis patients, symptom misattribution was associated with increased grey matter in the right and left caudate, right thalamus, left insula, putamen and cerebellum. In the chronic schizophrenia study, there were no significant associations between regional grey matter volume and measures of insight. These findings suggest that neuroplastic changes within subcortical and frontotemporal regions are associated with impaired insight in individuals during their first episode of psychosis.

Similar content being viewed by others
References
Amador X, David A (eds) (2004) Insight and Psychosis: awareness of illness in schizophrenia and related disorders, 2nd edn. Oxford University Press, London
Amador X, Strauss D (1993) Poor insight in schizophrenia. Psychiatr Q 64:305–318
Pia L, Tamietto M (2006) Unawareness in schizophrenia: neuropsychological and neuroanatomical findings. Psychiatry Clin Neurosci 60:531–537
Aleman A, Agrawal N, Morgan KD, David AS (2006) Insight in psychosis and neuropsychological function: meta-analysis. Br J Psychiatry 189:204–212
Cooke MA, Peters ER, Kuipers E, Kumari V (2005) Disease, deficit or denial? Models of poor insight in psychosis. Acta Psychiatr Scand 112:4–17
Sapara A, Cooke M, Fannon D, Francis A, Buchanan RW, Anilkumar AP, Barkataki I, Aasen I, Kuipers E, Kumari V (2007) Prefrontal cortex and insight in schizophrenia: a volumetric MRI study. Schizophr Res 89:22–34
Laroi F, Fannemel M, Ronneberg U, Flekkoy K, Opjordsmoen S, Dullerud R, Haakonsen M (2000) Unawareness of illness in chronic schizophrenia and its relationship to structural brain measures and neuropsychological tests. Psychiatry Res 100:49–58
Flashman LA, McAllister TW, Johnson SC, Rick JH, Green RL, Saykin AJ (2001) Specific frontal lobe subregions correlated with unawareness of illness in schizophrenia: a preliminary study. J Neuropsychiatry Clin Neurosci 13:255–257
Shad MU, Muddasani S, Prasad K, Sweeney JA, Keshavan MS (2004) Insight and prefrontal cortex in first-episode Schizophrenia. Neuroimage 22:1315–1320
Shad MU, Muddasani S, Keshavan MS (2006) Prefrontal subregions and dimensions of insight in first-episode schizophrenia–a pilot study. Psychiatry Res 146:35–42
Cooke MA, Fannon D, Kuipers E, Peters E, Williams SC, Kumari V (2008) Neurological basis of poor insight in psychosis: a voxel-based MRI study. Schizophr Res 103:40–51
Ha TH, Youn T, Ha KS, Rho KS, Lee JM, Kim IY, Kim SI, Kwon JS (2004) Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res 132:251–260
Bassitt DP, Neto MR, de Castro CC, Busatto GF (2007) Insight and regional brain volumes in schizophrenia. Eur Arch Psychiatry Clin Neurosc 257:58–62
Morgan KD, Dazzan P, Morgan C, Lappin J, Hutchinson G, Suckling J, Fearon P, Jones PB, Leff J, Murray RM, David AS (2010) Insight, grey matter and cognitive function in first-onset psychosis. Br J Psychiatry 197:141–148
Spitzer RL, Williams JB, Gibbon M (1994) Structured clinical interview for DSM-IV-patient version (SCID-P). New York State Psychiatric Institute, New York
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Beiser M, Erickson D, Fleming JA, Iacono WG (1993) Establishing the onset of psychotic illness. Am J Psychiatry 150:1349–1354
Kemp R, David AS (1997) Insight and compliance. In: Blackwell B (ed) Treatment compliance and the therapeutic alliance in serious mental illness. Harwood Academic Publishers, The Netherlands, pp 61–84
Amador XF, Strauss DH, Yale SA, Flaum MM, Endicott J, Gorman JM (1993) Assessment of insight in psychosis. Am J Psychiatry 150:873–879
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Ashburner J, Friston KJ (2001) Comments and controversies—why voxel-based morphometry should be used. Neuroimage 14:1238–1243
Nichols TE, Holmes AP (2001) Nonparametric analysis of PET functional neuroimaging experiments: a primer. Human Brain Mapp 15:1–25
Moorhead WJ, Job DE, Whalley HC, Sanderson TL, Johnstone EC, Lawrie SM (2004) Voxel-based morphometry of comorbid schizophrenia and learning disability: analyses in normalized and native spaces using parametric and nonparametric statistical methods. Neuroimage 22:188–202
Chumbley JR, Friston KJ (2009) False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage 44:62–70
Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26:839–851
Looi JC, Lindberg O, Liberg B, Tatham V, Kumar R, Maller J, Millard E, Sachdev P, Hogberg G, Pagani M (2008) Volumetrics of the caudate nucleus: reliability and validity of a new manual tracing protocol. Psychiatry Res 163:279–288
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128
Rossell SL, Coakes J, Shapleske J, Woodruff PW, David AS (2003) Insight: its relationship with cognitive function, brain volume, and symptoms in schizophrenia. Psychol Med 33:111–119
David A, van Os J, Jones P, Harvey I, Foerster A, Fahy T (1995) Insight and psychotic illness. Cross-sectional and longitudinal associations. Br J Psychiatry 167:621–628
David AS (1990) On insight and psychosis: discussion paper. J R Soc Med 83:325–329
Thompson KN, McGorry PD, Harrigan SM (2001) Reduced awareness of illness in first-episode psychosis. Compr Psychiatry 42:498–503
Takahashi H, Matsuura M, Koeda M, Yahata N, Suhara T, Kato M, Okubo Y (2008) Brain activations during judgments of positive self-conscious emotion and positive basic emotion: pride and joy. Cereb Cortex 18:898–903
Farrer C, Frith CD (2002) Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage 15:596–603
Modinos G, Ormel J, Aleman A (2009) Activation of anterior insula during self-reflection. PLoS ONE 4:e4618
Karnath HO, Baier B, Nagele T (2005) Awareness of the functioning of one’s own limbs mediated by the insular cortex? J Neurosci 25:7134–7138
Pia L, Neppi-Modona M, Ricci R, Berti A (2004) The anatomy of anosognosia for hemiplegia: a meta-analysis. Cortex 40:367–377
Cummings JL (1993) Frontal-subcortical circuits and human behavior. Arch Neurol 50:873–880
Mendrek A, Laurens KR, Kiehl KA, Ngan ET, Stip E, Liddle PF (2004) Changes in distributed neural circuitry function in patients with first-episode schizophrenia. Br J Psychiatry 185:205–214
Wager TD, Smith EE (2003) Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci 3:255–274
Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S (1999) Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry 45:1128–1137
Joel D, Weiner I (2000) The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 96:451–474
Goldberg G (1985) Supplementary motor area structure and function: review and hypotheses. Behav Brain Sci 8:567–616
Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS (2003) Human striatal response to salient non rewarding stimuli. J Neurosci 23:8092–8097
Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M (1999) Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry 46:908–920
Picard H, Amado I, Mouchet-Mages S, Olie JP, Krebs MO (2008) The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull 34:155–172
Nitschke MF, Stavrou G, Melchert UH, Erdmann C, Petersen D, Wessel K, Heide W (2003) Modulation of cerebellar activation by predictive and non-predictive sequential finger movements. Cerebellum 2:233–240
Elliott R, Dolan RJ (1998) Activation of different anterior cingulate foci in association with hypothesis testing and response selection. Neuroimage 8:17–29
Shergill SS, Brammer MJ, Fukuda R, Williams SC, Murray RM, McGuire PK (2003) Engagement of brain areas implicated in processing inner speech in people with auditory hallucinations. Br J Psychiatry 182:525–531
Schmidt H, McFarland J, Ahmed M, Elliott M, Cannon D, McDonald C (2010) Grey matter deficits in Chronic Schizophrenia not present at First Episode. Schizophr Res Suppl 2:460
Shad MU, Keshavan MS, Tamminga CA, Cullum CM, David A (2007) Neurobiological underpinnings of insight deficits in schizophrenia. Int Rev Psychiatry 19:437–446
Buchy L, Ad-Dab’bagh Y, Malla A, Lepage C, Bodnar M, Joober R, Sergerie K, Evans A, Lepage M (2011) Cortical thickness is associated with poor insight in first-episode psychosis. J Psychiat Res 45:781–787
Antonius D et al (2011) White matter integrity and lack of insight in schizophrenia and schizoaffective disorder. Schizophr Res
Kapur S (2003) Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 160:13–23
Delago MRS, Fiez JA (2004) Motivation-dependent responses in the human caudate nucleus. Cereb Cortex 14:1022–1030
Cleghorn JM, Franco S, Szechtman B, Kaplan RD, Szechtman H, Brown GM, Nahmias C, Garnett ES (1992) Toward a brain map of auditory hallucinations. Am J Psychiatry 149:1062–1069
Chambers RA, Potenza MN, Hoffman RE, Miranker W (2004) Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology 29:747–758
Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A (2006) Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci 26:6314–6317
Ramsden S, Richardson FM, Josse G, Thomas MSC, Ellis C, Shakeshaft C, Seghier ML, Price CJ (2011) Verbal and non-verbal intelligence changes in the teenage brain. Nature 479:113–116
Acknowledgments
Our thanks to Geraldine Dowd and radiography colleagues from the Dept. of Radiology, University Hospital Galway, for MRI data acquisition and to John Newell, Senior Lecturer in Biostatistics, Clinical Research Facility Galway, for statistical advice. This study was supported in part by an unrestricted research grant from Astra Zeneca.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McFarland, J., Cannon, D.M., Schmidt, H. et al. Association of grey matter volume deviation with insight impairment in first-episode affective and non-affective psychosis. Eur Arch Psychiatry Clin Neurosci 263, 133–141 (2013). https://doi.org/10.1007/s00406-012-0333-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-012-0333-8