Abstract
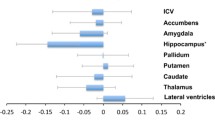
Structural changes in subcortical nuclei may underlie clinical symptoms of mood disorders. The goal was to determine whether macrostructural changes exist in brain areas assumed to be involved in regulation of mood and whether such changes differ between major depressive disorder and bipolar disorder. A case–control design was used to compare volumes of all major subcortical nuclei. Brains of patients with major depressive disorder (n = 9) or bipolar disorder (n = 11) or of individuals without a neuropsychiatric disorder (n = 22) were included. Exclusion criteria were a history of substance abuse or histological signs of neurodegenerative disorders.Volumes of the striato–pallidal nuclei, of the hypothalamus, thalamus, amygdala, hippocampus and basal limbic forebrain were determined in the right and left hemisphere by planimetry of 20 μm whole brain serial paraffin sections. Comparisons between patients with bipolar disorder, major depressive disorder and controls showed a significant (Λ = 0.35, F20,56 = 1.93, P = 0.028) overall difference in volumes of all investigated regions with strong effect sizes ( ƒ > 0.40) contributed by the hypothalamus, external pallidum, putamen and thalamus. As compared to controls, a strong effect size (ƒ > 0.40) was found in the bipolar group for smaller volumes of the hypothalamus, external pallidum, putamen and thalamus,whereas in patients with major depressive disorder a strong effect size was only found for a smaller volume of the external pallidum. In conclusion our data suggest that pathways presumably involved in mood regulation have structural pathology in affective disorders with more pronounced abnormalities in bipolar disorder.
Similar content being viewed by others
References
Alexander GE, Deleing MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J (1998) Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry 55:663–664
Ashtari M, Greenwald BS, Kramer–Ginsberg E,Hu J,Wu H, Patel M,Aupperle P,Pollack S (1999) Hippocampal/amygdala volumes in geriatric depression. Psychol Medicine 29:629–638
Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ, Nemeroff CB, Ellinwood EH Jr, Krishnan KR (1993) Hypercortisolemia and hippocampal changes in depression. Psychiatry Res 47:163–173
Aylward EH, Roberts–Twillie JV, Barta PE, Kumar AJ, Harris GJ, Geer M, Peyser CE, Pearlson GD (1994) Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am J Psychiatry 151:687–693
Baumann B, Bogerts B (1999) The pathomorphology of schizophrenia and mood disorders: similarities and differences. Schizophr Res 39:141–148
Baumann B, Bogerts B (2001) Neuroanatomical studies on bipolar disorder. Br J Psychiatry 41:142–147
Baumann B, Bornschlegl C,Krell D, Bogerts B (1997) Changes in CSF spaces differ in endogenous and neurotic depression. A planimetric CT scan study. J Affect Disord 45:179–188
Baumann B, Danos P,Krell D,Diekmann S, Leschinger A, Stauch R, Wurthmann C, Bernstein HG, Bogerts B (1999) Reduced volume of limbic system–affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatry Clin Neurosci 11:71–78
Bernstein HG,Heinemann A,Krell D,Mawrin C,Bielau H,Danos P, Diekmann S,Keilhoff G, Bogerts B, Baumann B (2002) Further immunohistochemical evidence for impaired NO–signaling in the hypothalamus of depressed patients. Ann N Y Acad Sci 973:91–93
Bernstein HG, Stanarius A, Baumann B, Henning H, Krell D, Danos P, Falkai P, Bogerts B (1998) Nitric oxide synthase–containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience 83:867–875
Beyer JL, Kuchibhatla M, Payne M, Moo–Young M, Cassidy F, MacFall J,Krishnan KR (2004) Caudate volume measurement in older adults with bipolar disorder. Int J Geriatr Psychiatry 19:109–114
Blumberg HP,Kaufman J,Martin A,Whiteman R,Zhang JH,Gore JC, Charney DS, Krystal JH, Peterson BS (2003) Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry 60:1201–1208
Bogerts B, Meertz E, Schonfeldt–Bausch R (1985) Basal ganglia and limbic system pathology in schizophrenia.A morphometric study of brain volume and shrinkage. Arch Gen Psychiatry 42:784–791
Brambilla P, Harenski K, Nicoletti MA, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC (2001) Anatomical MRI study of basal ganglia in bipolar disorder patients. Psychiatry Res 106:65–80
Brambilla P,Nicoletti M, Sassi RB,Mallinger AG, Frank E,Keshavan MS,Soares JC (2004) Corpus callosum signal intensity in patients with bipolar and unipolar disorder. J Neurol Neurosurg Psychiatry 75:221–225
Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS (2000) Hippocampal volume reduction in major depression. Am J Psychiatry 57:115–118
Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS (2002) Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry 51:273–279
Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B,Wu H, Kinon B,Ashtari M (1994) Increase in caudate nuclei volumes of first–episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 151:1430–1436
Coffey CE, Wilkinson WE, Weiner RD, Parashos IA, Djang WT, Webb MC, Figiel GS, Spritzer CE (1993) Quantitative cerebral anatomy in depression. A controlled magnetic resonance imaging study. Arch Gen Psychiatry 50:7–16
Coffman JA ,Bornstein RA, Olson SC, Schwarzkopf SB, Nasrallah HA (1990) Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biol Psychiatry 27:1188–1196
Dasari M, Friedman L, Jesberger J, Stuve TA, Findling RL, Swales TP, Schulz SC (1999) A magnetic resonance imaging study of thalamic area in adolescent patients with either schizophrenia or bipolar disorder as compared to healthy controls.Psychiatry Res 91:155–162
Dewulf A (1972) Anatomy of the Normal Human Thalamus, Topometry, and Standardized Nomenclature. Elsevier, Amsterdam
Drevets WC,Ongür D, Price JL (1998) Reduced glucose metabolism in the subgenual prefrontal cortex in unipolar depression. Mol Psychiatry 3:190–191
Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME (1997) Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386:824–827
Driessen M, Herrmann J, Stahl K, Zwaan M ,Meier S, Hill A, Osterheider M, Petersen D (2000) Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry 57:1115–1122
Dupont RM, Jernigan TL, Heindel W, Butters N, Shafer K, Wilson T, Hesselink J, Gillin JC (1995) Magnetic resonance imaging and mood disorders. Localization of white matter and other subcortical abnormalities. Arch Gen Psychiatry 52:747–755
Elkis H, Friedman L, Wise A, Meltzer HY (1995) Meta–analyses of studies of ventricular enlargement and cortical sulcal prominence in mood disorders. Comparisons with controls or patients with schizophrenia. Arch Gen Psychiatry 52:735–746
Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, Jager M, Leinsinger G, Hahn K, Moller HJ (2002a) Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry 51:708–714
Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, Leinsinger G, Bottlender R, Hahn K, Moller HJ (2002b) Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 159:1112–1118
Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, Bottlender R, Leinsinger G, Moller HJ (2003) Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry 53:338–344
Frodl T, Meisenzahl EM, Zetzsche T,Hohne T, Banac S, Schorr C, Jager M, Leinsinger G, Bottlender R, Reiser M, Moller HJ (2004) Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1–year follow–up. J Clin Psychiatry 65:492–499
Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ (2004) Volumetric analysis of the prefrontal cortex,amygdala, and hippocampus in major depression. Neuropsychopharmacology 29: 952–959
Hauser P, Matochik J, Altshuler LL, Denicoff KD, Conrad A, Li X, Post RM (2000) MRI–based measurements of temporal lobe and ventricular structures in patients with bipolar I and bipolar II disorders. J Affect Disord 60:25–32
Herrmann M, Bartels C, Schumacher M, Wallesch CW (1995) Poststroke depression. Is there a pathoanatomic correlate for depression in the postacute stage of stroke? Stroke 26:850–856
Hirai T, Jones EG (1989) A new parcellation of the human thalamus on the basis of histochemical staining. Brain Res Brain Res Rev 14:1–34
Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, Yurgelun–Todd D, Zarate C, Kikinis R, Jolesz FA, McCarley RW (1999) Subgenual cingulate cortex volume in firstepisode psychosis. Am J Psychiatry 156:1091–1093
Holsboer F, Barden N (1996) Antidepressants and hypothalamicpituitary– adrenocortical regulation. Endocr Rev 17:187–205
Husain MM, McDonald WM, Doraiswamy PM, Figiel GS, Na C, Escalona PR, Boyko OB, Nemeroff CB, Krishnan KR (1991) A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res 40:95–99
Iacono WG, Smith GN,Moreau M, Beiser M, Fleming JA, Lin TY, Flak B (1988) Ventricular and sulcal size at the onset of psychosis. Am J Psychiatry 145:820–824
Krishnan KR, McDonald WM, Escalona PR, Doraiswamy PM, Na C, Husain MM, Figiel GS, Boyko OB, Ellinwood EH, Nemeroff CB (1992) Magnetic resonance imaging of the caudate nuclei in depression. Preliminary observations. Arch Gen Psychiatry 49:553–557
Lopez–Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM (2002) Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry 52:93–100
McDonald C, Zanelli J, Rabe–Hesketh S, Ellison–Wright I, Sham P, Kalidindi S, Murray RM, Kennedy N (2004) Meta–analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry 56:411–417
Mervaala E, Fohr J, Kononen M,Valkonen–Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamaki H, Karjalainen A K, Lehtonen J (2000) Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 30:117–125
Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK (2000) Lithium–induced increase in human brain grey matter. Lancet 356:1241–1242
Nakano K, Kayahara T, Tsutsumi T, Ushiro H (2000) Neural circuits and functional organization of the striatum. J Neurology 247:1–15
Ongür D, Drevets WC, Price JL (1998) Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA 95:13290–13295
Parashos IA, Tupler LA, Blitchington T, Krishnan KR (1998) Magnetic– resonance morphometry in patients with major depression. Psychiatry Res 84:7–15
Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF (1996) Increased number of vasopressin– and oxytocin–expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry 53:137–143
Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF (1995) Corticotropin–releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry 152:1372–1376
Rajkowska G, Halaris A, Selemon LD (2001) Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry 49:741–752
Rajkowska G, Miguel–Hidalgo JJ, Wie J, Dilley G, Pittman SD, Meltzer HY,Overholser JC,Roth BL, Stockmeier CA (1999) Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45:1085–1098
Sapolsky RM (2000) Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 57:925–935
Sassi RB, Nicoletti M, Brambilla P, Harenski K, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC (2001) Decreased pituitary volume in patients with bipolar disorder. Biol Psychiatry 50:271–280
Sheline YI, Gado MH, Price JL (1998) Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport 9:2023–2028
Sheline YI, Sanghavi M, Mintun MA, Gado MH (1999) Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neuroscience 19:5034–5043
Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW (1996) Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 93:3908–3913
Soares JC, Mann JJ (1997) The anatomy of mood disorders–review of structural neuroimaging studies. Biol Psychiatry 41:86–106
Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF, MacFall JR, Krishnan KR (2000) Hippocampal volume in geriatric depression. Biol Psychiatry 48:301–309
Stevens J (1990) Intermediate Statistics: A Modern Approach. Lawrence Erlbaum Associates Inc,Hillsdale, NJ
Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER (1999) Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry 56:254–260
Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J, Shear P, Adler CM (2002) Ventricular and periventricular structural volumes in first– versus multiple–episode bipolar disorder. Am J Psychiatry 159:1841–1847
Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello–Cintron CM,Yurgelun–Todd DA (2000) Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry 47:1087–1090
Vataja R, Pohjasvaara T, Leppavuori A, Mantyla R, Aronen HJ, Salonen O, Kaste M, Erkinjuntti T (2001) Magnetic resonance imaging correlates of depression after ischemic stroke. Arch Gen Psychiatry 58:925–931
Vawter MP, Freed WJ, Kleinman JE (2000) Neuropathology of bipolar disorder. Biol Psychiatry 48:486–504
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bielau, H., Trübner, K., Krell, D. et al. Volume deficits of subcortical nuclei in mood disorders. Eur Arch Psychiatry Clin Neurosci 255, 401–412 (2005). https://doi.org/10.1007/s00406-005-0581-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-005-0581-y