Abstract
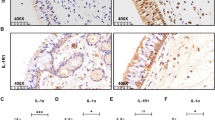
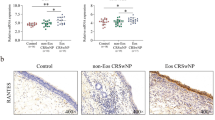
Interleukin 17C (IL-17C) is a functionally distinct member of the IL-17 family that is selectively induced in epithelia by bacterial challenge and inflammatory stimuli. The goal of this study was to explore the expression of IL-17C in nasal epithelial cells and their role in the pathogenesis of chronic rhinosinusitis with nasal polyposis (CRSwNPs). IL-17C expression was detected using immunohistochemistry (IHC) of the epithelial cell layers and using the western blot assay on whole tissue homogenates from control subjects (n = 10) and CRSwNP patients [10 non-eosinophilic polyps and 10 eosinophilic polyps (EPs)]. Expression of IL-17C and P47-phox were evaluated in the human nasal epithelial cells (RPMI-2650 cells) after treatment with staphylococcal enterotoxin B (SEB) and pretreatment with reactive oxygen species (ROS) scavenger, N-acetyl l-cysteine (NAC). Finally, IL-17C expression was demonstrated in eosinophilic rhinosinusitis murine model using IHC. Epithelial expression of IL-17C was higher in nasal polyps (especially in EPs) compared to control mucosa. SEB increased the expression of IL-17C and P47-phox in RPMI-2650 cells. SEB-induced expressions of both IL-17C and P47-phox were significantly decreased in NAC-pretreated cells. Epithelial expression of IL-17C was significantly higher in experimental mice compared to control mice. SEB-induced IL-17C expression in nasal epithelial cells is mediated by ROS production. This pathway may be associated with the pathogenesis of CRSwNP, especially eosinophilic nasal polyps.







Similar content being viewed by others
References
Fokkens WJ, Lund VJ, Mullol J et al (2012) European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 3p preceding table of contents, 1-298
Corriveau MN, Zhang N, Holtappels G, Van Roy N, Bachert C (2009) Detection of Staphylococcus aureus in nasal tissue with peptide nucleic acid-fluorescence in situ hybridization. Am J Rhinol Allerg 23:461–465
Sachse F, Becker K, von Eiff C, Metze D, Rudack C (2010) Staphylococcus aureus invades the epithelium in nasal polyposis and induces IL-6 in nasal epithelial cells in vitro. Allergy 65:1430–1437
Bernstein JM, Ballow M, Schlievert PM, Rich G, Allen C, Dryja D (2003) A superantigen hypothesis for the pathogenesis of chronic hyperplastic sinusitis with massive nasal polyposis. Am J Rhinol 17:321–326
Van Zele T, Gevaert P, Watelet JB et al (2004) Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol 114:981–983
Yu RL, Dong Z (2009) Proinflammatory impact of Staphylococcus aureus enterotoxin B on human nasal epithelial cells and inhibition by dexamethasone. Am J Rhinol Allergy 23:15–20
O’Brien GJ, Riddell G, Elborn JS, Ennis M, Skibinski G (2006) Staphylococcus aureus enterotoxins induce IL-8 secretion by human nasal epithelial cells. Respir Res 7:115
Huvenne W, Callebaut I, Reekmans K et al (2010) Staphylococcus aureus enterotoxin B augments granulocyte migration and survival via airway epithelial cell activation. Allergy 65:1013–1020
Kim DW, Khalmuratova R, Hur DG et al (2011) Staphylococcus aureus enterotoxin B contributes to induction of nasal polypoid lesions in an allergic rhinosinusitis murine model. Am J Rhinol Allerg 25:e255–e261
Chang SH, Reynolds JM, Pappu BP, Chen G, Martinez GJ, Dong C (2011) Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity 35:611–621
Gaffen SL (2009) Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9:556–567
Pfeifer P, Voss M, Wonnenberg B et al (2013) IL-17C is a mediator of respiratory epithelial innate immune response. Am J Respir Cell Mol Biol 48:415–421
Ramirez-Carrozzi V, Sambandam A, Luis E et al (2011) IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol 12:1159–1166
Im E, Jung J, Rhee SH (2012) Toll-like receptor 5 engagement induces interleukin-17C expression in intestinal epithelial cells. J Interferon Cytokine Res 32:583–591
Johnston A, Fritz Y, Dawes SM et al (2013) Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol 190:2252–2262
Cao PP, Li HB, Wang BF et al (2009) Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allerg Clin Immunol 124:478–484 e471–472
Boldogh I, Bacsi A, Choudhury BK et al (2005) ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest 115:2169–2179
Kedzierska A, Kaszuba-Zwoinska J, Slodowska-Hajduk Z et al (2005) SEB-induced T cell apoptosis in atopic patients–correlation to clinical status and skin colonization by Staphylococcus aureus. Arch Immunol Ther Exp (Warsz) 53:63–70
Uneri C, Ozturk O, Polat S, Yuksel M, Haklar G (2005) Determination of reactive oxygen species in nasal polyps. Rhinology 43:185–189
Krakauer T (2010) Therapeutic down-modulators of staphylococcal superantigen-induced inflammation and toxic shock. Toxins (Basel) 2:1963–1983
Grote K, Flach I, Luchtefeld M et al (2003) Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res 92:e80–e86
Xiao C, Puddicombe SM, Field S et al (2011) Defective epithelial barrier function in asthma. J Allerg Clin Immunol 128:549–556 e541–512
Beisswenger C, Lysenko ES, Weiser JN (2009) Early bacterial colonization induces toll-like receptor-dependent transforming growth factor beta signaling in the epithelium. Infect Immun 77:2212–2220
Jeanson L, Kelly M, Coste A et al (2012) Oxidative stress induces unfolding protein response and inflammation in nasal polyposis. Allergy 67:403–412
Segal BH, Han W, Bushey JJ et al (2010) NADPH oxidase limits innate immune responses in the lungs in mice. PLoS One 5:e9631
Acknowledgments
We thank the study participants and all joint research workers. We also thank the patients who provided samples for the study.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0009790).
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, J., Rha, KS., Kim, D.W. et al. IL-17C expression in nasal epithelial cells of chronic rhinosinusitis with nasal polyposis. Eur Arch Otorhinolaryngol 271, 1097–1105 (2014). https://doi.org/10.1007/s00405-013-2683-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-013-2683-x