Abstract
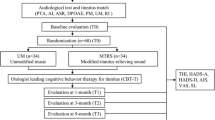
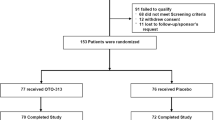
The aim of this study was to assess the effect of ondansetron on symptoms of patients with subjective tinnitus accompanied by sensorineural hearing loss or normal hearing. Sixty patients with a chief complaint of tinnitus (with duration of more than 3 months) were equally randomized to ondansetron or placebo for 4 weeks. The dose of ondansetron was gradually increased from 4 mg/day (one tablet) to 16 mg/day (4 tablets) during 12 days and then continued up to 4 weeks. The exact number of tablets was prescribed in the placebo group. Patients underwent audiologic examinations and filled questionnaires at baseline and after 4 weeks of treatment. Our primary outcomes were changes in Tinnitus Handicap Inventory questionnaire (THI), Tinnitus Severity Index (TSI) and visual analog scale (VAS) scores. Our secondary outcomes were the changes in depression and anxiety based on Hospital Anxiety and Depression (HADS) questionnaire, side effects, tinnitus loudness matching, tinnitus pitch matching, pure tone audiometry and speech recognition threshold (SRT). In the ondansetron and placebo groups, 27 and 26 patients completed the study, respectively. The changes in VAS (P = 0.934), THI (P = 0.776), anxiety (P = 0.313) and depression (P = 0.163) scores were not different between the groups. TSI score decreased significantly in the ondansetron compared with the placebo group (P = 0.004). Changes in tinnitus loudness matching (P = 0.75) and pitch matching (P = 0.56) did not differ between the two groups. Ondansetron, but not placebo, decreased the SRT threshold (right, P < 0.001; left, P = 0.043) and mean PTA (right, P = 0.006; left, P < 0.001). In conclusion, ondansetron reduces the severity of tinnitus hypothetically through cochlear amplification.

Similar content being viewed by others
References
Eggermont JJ (2003) Central tinnitus. Auris Nasus Larynx 30:7–12
Sanchez L (2004) The epidemiology of tinnitus. Audiol med 2(1):8–17
McFerran D, Phillips J (2007) Tinnitus. J Laryngol Otol 121(03):201–208
Vio MM, Holme RH (2005) Hearing loss and tinnitus: 250 million people and a US$10 billion potential market. Drug Discov Today 10(19):1263–1265
Chan Y (2009) Tinnitus: etiology, classification, characteristics, and treatment. Discov Med 8(42):133–136
Kleinjung T (2011) Surgical treatment: the ear. In: Moller AR, Landgrebe M, De Ridder D, Kleinjung T (eds) Text book of tinnitus. Springer, New York, p 663
Dobie RA (1999) A review of randomized clinical trials in tinnitus. Laryngoscope 109(8):1202–1211
Jos JE (2005) Tinnitus: neurobiological substrates. Drug Discov Today 10(19):1283–1290
Dallos P, Popper AN, Fay RR (1996) The cochlea. In: Springer handbook of auditory research, vol. 8. Springer, New York, pp 435–502
Zheng XY, Henderson D, McFadden SL, Ding DL, Salvi RJ (1999) Auditory nerve fiber responses following chronic cochlear de-efferentation. J Comp Neurol 406(1):72–86
Delano PH, Elgueda D, Hamame CM, Robles L (2007) Selective attention to visual stimuli reduces cochlear sensitivity in chinchillas. J Neurosci 27(15):4146–4153
Maison SF, Liberman MC (2000) Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci 20(12):4701–4707
Eggermont JJ (2005) Tinnitus: neurobiological substrates. Drug Discov Today 10(19):1283–1290
Van de Heyning P, Vermeire K, Diebl M, Nopp P, Anderson I, De Ridder D (2008) Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol 117(9):645–652
Rothlin CV, Lioudyno MI, Silbering AF, Plazas PV, Casati ME, Katz E, Guth PS, Elgoyhen AB (2003) Direct interaction of serotonin type 3 receptor ligands with recombinant and native alpha 9 alpha 10-containing nicotinic cholinergic receptors. Mol Pharmacol 63(5):1067–1074
Elgoyhen AB, Katz E, Fuchs PA (2009) The nicotinic receptor of cochlear hair cells: a possible pharmacotherapeutic target? Biochem Pharmacol 78(7):712–719
Newman CW, Jacobson GP, Spitzer JB (1996) Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg 122(2):143–148
Meikle M, Griest S, Stewart B, Press LS (1995) Measuring the negative impact of tinnitus: a brief severity index
Zigmond AS, Snaith RP (1983) The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 67(6):361–370
Henry JA, Zaugg TL, Schechter MA (2005) Clinical guide for audiologic tinnitus management II: treatment. Am J Audiol 14(1):49–70
Henry JA, Zaugg TL, Schechter MA (2005) Clinical guide for audiologic tinnitus management I: assessment. Am J Audiol 14(1):21–48
Langguth B, Goodey R, Azevedo A, Bjorne A, Cacace A, Crocetti A, Del Bo L, De Ridder D, Diges I, Elbert T, Flor H, Herraiz C, Ganz Sanchez T, Eichhammer P, Figueiredo R, Hajak G, Kleinjung T, Landgrebe M, Londero A, Lainez MJ, Mazzoli M, Meikle MB, Melcher J, Rauschecker JP, Sand PG, Struve M, Van de Heyning P, Van Dijk P, Vergara R (2007) Consensus for tinnitus patient assessment and treatment outcome measurement: tinnitus research initiative meeting, Regensburg, July 2006. Prog Brain Res 166:525–536
He Y (2010) Missing data analysis using multiple imputation: getting to the heart of the matter. Circ Cardiovasc Qual Outcomes 3(1):98–105
Langguth B, Kleinjung T, Fischer B, Hajak G, Eichhammer P, Sand PG (2007) Tinnitus severity, depression, and the big five personality traits. Prog Brain Res 166:221–225
Landgrebe M, Langguth B, Zeman F, Koller M (2011) Methodology of clinical trials for tinnitus. In: Moller AR, Landgrebe M, De Ridder D, Kleinjung T (eds) Text book of tinnitus. Springer, New York, pp 202–203
Simmons DD, Morley BJ (1998) Differential expression of the alpha 9 nicotinic acetylcholine receptor subunit in neonatal and adult cochlear hair cells. Brain Res Mol Brain Res 56(1–2):287–292
Del Bo L, Ambrosetti U (2007) Hearing aids for the treatment of tinnitus. Prog Brain Res 166:341–345
Gelfand SA (2009) Speech audiometry. In: Gelfand SA (ed) Essentials of audiology, 3rd edn. Thieme Medical Publisher, New York, p 241
Meikle MB, Henry JA, Griest SE, Stewart BJ, Abrams HB, McArdle R, Myers PJ, Newman CW, Sandridge S, Turk DC, Folmer RL, Frederick EJ, House JW, Jacobson GP, Kinney SE, Martin WH, Nagler SM, Reich GE, Searchfield G, Sweetow R, Vernon JA (2012) The Tinnitus Functional Index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear 32(2):153–176
Acknowledgments
This project was funded by the Tehran University of Medical Sciences, grant number 89-01-48-1045. The trial was registered in IRCT.ir (ID: IRCT201105185867N4). This project was MD-MPH thesis of the first author.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taslimi, S., Vahidi, H., Pourvaziri, A. et al. Ondansetron in patients with tinnitus: randomized double-blind placebo-controlled study. Eur Arch Otorhinolaryngol 270, 1635–1641 (2013). https://doi.org/10.1007/s00405-012-2179-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-012-2179-0