Abstract
Background
Determination of mutation status of BRCA1 and BRCA2 has become part of the clinical routine. However, the spectrum of genetic variants differs between populations. The aim of this study was to deliver a comprehensive description of all detected variants.
Methods
In families fulfilling one of the German Consortium for Hereditary Breast and Ovarian Cancer (GC-HBOC) criteria for genetic testing, one affected was chosen for analysis. DNA of blood lymphocytes was amplified by PCR and prescreened by DHPLC. Aberrant fragments were sequenced. All coding exons and splice sites of BRCA1 and BRCA2 were analyzed. Screening for large rearrangements in both genes was performed by MLPA.
Results
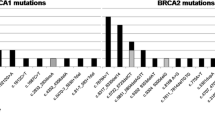
Of 523 index patients, 121 (23.1%) were found to carry a pathogenic or likely pathogenic (class 4/5) mutation. A variant of unknown significance (VUS) was detected in 73/523 patients (13.9%). Two mutations p.Gln1756Profs*74 and p.Cys61Gly comprised 42.3% (n = 33/78) of all detected pathogenic mutations in BRCA1. Most of the other mutations were unique mutations. The most frequently detected mutation in BRCA2 was p.Val1283Lys (13.9%; n = 6/43). Altogether, 101 different neutral genetic variants were counted in BRCA1 (n = 35) and in BRCA2 (n = 66).
Conclusion
The two most frequently detected mutations are founder mutations in Poland and Czech Republic. More similarities seem to be shared with our direct neighbor countries compared to other European countries. For comparison of the extended genotype, a shared database is needed.
Similar content being viewed by others
References
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T, Matei D, Macpherson E, Watkins C, Carmichael J, Matulonis U (2012) Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 366(15):1382–1392. doi:10.1056/NEJMoa1105535
Perez EA, Suman VJ, Davidson NE, Martino S, Kaufman PA, Lingle WL, Flynn PJ, Ingle JN, Visscher D, Jenkins RB (2006) HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol 24(19):3032–3038. doi:10.1200/jco.2005.03.4744
Meisel JL, Hyman DM, Garg K, Zhou Q, Dao F, Bisogna M, Gao J, Schultz ND, Grisham RN, Phillips M, Iasonos A, Kauff ND, Levine DA, Soslow RA, Spriggs DR (2014) The performance of BRCA1 immunohistochemistry for detecting germline, somatic, and epigenetic BRCA1 loss in high-grade serous ovarian cancer. Ann Oncol 25(12):2372–2378. doi:10.1093/annonc/mdu461
Gorski B, Jakubowska A, Huzarski T, Byrski T, Gronwald J, Grzybowska E, Mackiewicz A, Stawicka M, Bebenek M, Sorokin D, Fiszer-Maliszewska L, Haus O, Janiszewska H, Niepsuj S, Gozdz S, Zaremba L, Posmyk M, Pluzanska M, Kilar E, Czudowska D, Wasko B, Miturski R, Kowalczyk JR, Urbanski K, Szwiec M, Koc J, Debniak B, Rozmiarek A, Debniak T, Cybulski C, Kowalska E, Toloczko-Grabarek A, Zajaczek S, Menkiszak J, Medrek K, Masojc B, Mierzejewski M, Narod SA, Lubinski J (2004) A high proportion of founder BRCA1 mutations in Polish breast cancer families. Int J Cancer 110(5):683–686. doi:10.1002/ijc.20162
Lievre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouche O, Landi B, Louvet C, Andre T, Bibeau F, Diebold MD, Rougier P, Ducreux M, Tomasic G, Emile JF, Penault-Llorca F, Laurent-Puig P (2008) KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26(3):374–379. doi:10.1200/jco.2007.12.5906
Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B, Gumpper KL, Scholl T, Tavtigian SV, Pruss DR, Critchfield GC (2002) Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol 20(6):1480–1490
Engert S, Wappenschmidt B, Betz B, Kast K, Kutsche M, Hellebrand H, Goecke TO, Kiechle M, Niederacher D, Schmutzler RK, Meindl A (2008) MLPA screening in the BRCA1 gene from 1,506 German hereditary breast cancer cases: novel deletions, frequent involvement of exon 17, and occurrence in single early-onset cases. Hum Mutat 29(7):948–958. doi:10.1002/humu.20723
Sluiter MD, van Rensburg EJ (2011) Large genomic rearrangements of the BRCA1 and BRCA2 genes: review of the literature and report of a novel BRCA1 mutation. Breast Cancer Res Treat 125(2):325–349. doi:10.1007/s10549-010-0817-z
Weitzel JN, Lagos V, Blazer KR, Nelson R, Ricker C, Herzog J, McGuire C, Neuhausen S (2005) Prevalence of BRCA mutations and founder effect in high-risk Hispanic families. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Int Soc Cell 14(7):1666–1671. doi:10.1158/1055-9965.epi-05-0072
Easton DF, Deffenbaugh AM, Pruss D, Frye C, Wenstrup RJ, Allen-Brady K, Tavtigian SV, Monteiro AN, Iversen ES, Couch FJ, Goldgar DE (2007) A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer-predisposition genes. Am J Hum Genet 81(5):873–883. doi:10.1086/521032
Spurdle AB, Healey S, Devereau A, Hogervorst FB, Monteiro AN, Nathanson KL, Radice P, Stoppa-Lyonnet D, Tavtigian S, Wappenschmidt B, Couch FJ, Goldgar DE (2012) ENIGMA—evidence-based network for the interpretation of germline mutant alleles: an international initiative to evaluate risk and clinical significance associated with sequence variation in BRCA1 and BRCA2 genes. Hum Mutat 33(1):2–7. doi:10.1002/humu.21628
Eccles DM, Mitchell G, Monteiro AN, Schmutzler R, Couch FJ, Spurdle AB, Gomez-Garcia EB (2015) BRCA1 and BRCA2 genetic testing-pitfalls and recommendations for managing variants of uncertain clinical significance. Ann Oncol 26(10):2057–2065. doi:10.1093/annonc/mdv278
Kast K, Rhiem K, Wappenschmidt B, Hahnen E, Hauke J, Bluemcke B, Zarghooni V, Herold N, Ditsch N, Kiechle M, Braun M, Fischer C, Dikow N, Schott S, Rahner N, Niederacher D, Fehm T, Gehrig A, Mueller-Reible C, Arnold N, Maass N, Borck G, de Gregorio N, Scholz C, Auber B, Varon-Manteeva R, Speiser D, Horvath J, Lichey N, Wimberger P, Stark S, Faust U, Weber BH, Emons G, Zachariae S, Meindl A, Schmutzler RK, Engel C (2016) Prevalence of BRCA1/2 germline mutations in 21 401 families with breast and ovarian cancer. J Med Genet. doi:10.1136/jmedgenet-2015-103672
Arnold N, Gross E, Schwarz-Boeger U, Pfisterer J, Jonat W, Kiechle M (1999) A highly sensitive, fast, and economical technique for mutation analysis in hereditary breast and ovarian cancers. Hum Mutat 14(4):333–339. doi:10.1002/(sici)1098-1004(199910)14:4<333::aid-humu9>3.0.co;2-c
Gross E, Arnold N, Pfeifer K, Bandick K, Kiechle M (2000) Identification of specific BRCA1 and BRCA2 variants by DHPLC. Hum Mutat 16 (4):345–353. doi:10.1002/1098-1004(200010)16:4<345::aid-humu7>3.0.co;2-#
Homig-Holzel C, Savola S (2012) Multiplex ligation-dependent probe amplification (MLPA) in tumor diagnostics and prognostics. Diagn Mol Pathol 21(4):189–206. doi:10.1097/PDM.0b013e3182595516
Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS, Hogervorst FB, Hoogerbrugge N, Spurdle AB, Tavtigian SV (2008) Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat 29(11):1282–1291. doi:10.1002/humu.20880
Kast K, Schmutzler RK, Rhiem K, Kiechle M, Fischer C, Niederacher D, Arnold N, Grimm T, Speiser D, Schlegelberger B, Varga D, Horvath J, Beer M, Briest S, Meindl A, Engel C (2014) Validation of the Manchester scoring system for predicting BRCA1/2 mutations in 9,390 families suspected of having hereditary breast and ovarian cancer. Int J Cancer. doi:10.1002/ijc.28875
Peto J, Collins N, Barfoot R, Seal S, Warren W, Rahman N, Easton DF, Evans C, Deacon J, Stratton MR (1999) Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 91(11):943–949
Anglian Breast Cancer Study Group (2000). Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer 83(10):1301–1308. doi:10.1054/bjoc.2000.1407
Suter NM, Ray RM, Hu YW, Lin MG, Porter P, Gao DL, Zaucha RE, Iwasaki LM, Sabacan LP, Langlois MC, Thomas DB, Ostrander EA (2004) BRCA1 and BRCA2 mutations in women from Shanghai China. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Int Soc Cell 13(2):181–189
Zhang J, Sun J, Chen J, Yao L, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Xie Y (2016) Comprehensive analysis of BRCA1 and BRCA2 germline mutations in a large cohort of 5931 Chinese women with breast cancer. Breast Cancer Res Treat. doi:10.1007/s10549-016-3902-0
Machackova E, Foretova L, Lukesova M, Vasickova P, Navratilova M, Coene I, Pavlu H, Kosinova V, Kuklova J, Claes K (2008) Spectrum and characterisation of BRCA1 and BRCA2 deleterious mutations in high-risk Czech patients with breast and/or ovarian cancer. BMC Cancer 8:140. doi:10.1186/1471-2407-8-140
Thorlacius S, Olafsdottir G, Tryggvadottir L, Neuhausen S, Jonasson JG, Tavtigian SV, Tulinius H, Ogmundsdottir HM, Eyfjord JE (1996) A single BRCA2 mutation in male and female breast cancer families from Iceland with varied cancer phenotypes. Nat Genet 13(1):117–119. doi:10.1038/ng0596-117
Meindl A (2002) Comprehensive analysis of 989 patients with breast or ovarian cancer provides BRCA1 and BRCA2 mutation profiles and frequencies for the German population. Int J Cancer 97(4):472–480
Caputo S, Benboudjema L, Sinilnikova O, Rouleau E, Beroud C, Lidereau R (2012) Description and analysis of genetic variants in French hereditary breast and ovarian cancer families recorded in the UMD-BRCA1/BRCA2 databases. Nucleic Acids Res 40(Database issue):D992–D1002. doi:10.1093/nar/gkr1160
Claes K, Poppe B, Coene I, Paepe AD, Messiaen L (2004) BRCA1 and BRCA2 germline mutation spectrum and frequencies in Belgian breast/ovarian cancer families. Br J Cancer 90(6):1244–1251. doi:10.1038/sj.bjc.6601656
Hansen T, Jonson L, Albrechtsen A, Andersen MK, Ejlertsen B, Nielsen FC (2009) Large BRCA1 and BRCA2 genomic rearrangements in Danish high risk breast-ovarian cancer families. Breast Cancer Res Treat 115(2):315–323. doi:10.1007/s10549-008-0088-0
Nielsen HR, Nilbert M, Petersen J, Ladelund S, Thomassen M, Pedersen IS, Hansen TV, Skytte AB, Borg A, Therkildsen C (2016) BRCA1/BRCA2 founder mutations and cancer risks: impact in the western Danish population. Fam Cancer. doi:10.1007/s10689-016-9875-7
Cipollini G, Tommasi S, Paradiso A, Aretini P, Bonatti F, Brunetti I, Bruno M, Lombardi G, Schittulli F, Sensi E, Tancredi M, Bevilacqua G, Caligo MA (2004) Genetic alterations in hereditary breast cancer. Ann Oncol 15(Suppl 1):I7–I13. doi:10.1093/annonc/mdh651
Coppa A, Buffone A, Capalbo C, Nicolussi A, D’Inzeo S, Belardinilli F, Colicchia V, Petroni M, Granato T, Midulla C, Zani M, Ferraro S, Screpanti I, Gulino A, Giannini G (2014) Novel and recurrent BRCA2 mutations in Italian breast/ovarian cancer families widen the ovarian cancer cluster region boundaries to exons 13 and 14. Breast Cancer Res Treat 148(3):629–635. doi:10.1007/s10549-014-3196-z
Vos JR, Teixeira N, van der Kolk DM, Mourits MJ, Rookus MA, van Leeuwen FE, Collee M, van Asperen CJ, Mensenkamp AR, Ausems MG, van Os TA, Meijers-Heijboer HE, Gomez-Garcia EB, Vasen HF, Brohet RM, van der Hout AH, Jansen L, Oosterwijk JC, de Bock GH (2014) Variation in mutation spectrum partly explains regional differences in the breast cancer risk of female BRCA mutation carriers in the Netherlands. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Int Soc Cell 23(11):2482–2491. doi:10.1158/1055-9965.epi-13-1279
Petrij-Bosch A, Peelen T, van Vliet M, van Eijk R, Olmer R, Drusedau M, Hogervorst FB, Hageman S, Arts PJ, Ligtenberg MJ, Meijers-Heijboer H, Klijn JG, Vasen HF, Cornelisse CJ, van ‘t Veer LJ, Bakker E, van Ommen GJ, Devilee P (1997) BRCA1 genomic deletions are major founder mutations in Dutch breast cancer patients. Nat Genet 17(3):341–345. doi:10.1038/ng1197-341
Janavicius R (2010) Founder BRCA1/2 mutations in the Europe: implications for hereditary breast-ovarian cancer prevention and control. EPMA J 1(3):397–412. doi:10.1007/s13167-010-0037-y
Hartmann C, John AL, Klaes R, Hofmann W, Bielen R, Koehler R, Janssen B, Bartram CR, Arnold N, Zschocke J (2004) Large BRCA1 gene deletions are found in 3% of German high-risk breast cancer families. Hum Mutat 24(6):534. doi:10.1002/humu.9291
Wojcik P, Jasiowka M, Strycharz E, Sobol M, Hodorowicz-Zaniewska D, Skotnicki P, Byrski T, Blecharz P, Marczyk E, Cedrych I, Jakubowicz J, Lubinski J, Sopik V, Narod S, Pierzchalski P (2016) Recurrent mutations of BRCA1, BRCA2 and PALB2 in the population of breast and ovarian cancer patients in Southern Poland. Hered Cancer Clin Pract 14:5. doi:10.1186/s13053-016-0046-5
Kirchhoff T, Gaudet MM, Antoniou AC, McGuffog L, Humphreys MK, Dunning AM, Bojesen SE, Nordestgaard BG, Flyger H, Kang D, Yoo KY, Noh DY, Ahn SH, Dork T, Schurmann P, Karstens JH, Hillemanns P, Couch FJ, Olson J, Vachon C, Wang X, Cox A, Brock I, Elliott G, Reed MW, Burwinkel B, Meindl A, Brauch H, Hamann U, Ko YD, Broeks A, Schmidt MK, Van ‘t Veer LJ, Braaf LM, Johnson N, Fletcher O, Gibson L, Peto J, Turnbull C, Seal S, Renwick A, Rahman N, Wu PE, Yu JC, Hsiung CN, Shen CY, Southey MC, Hopper JL, Hammet F, Van Dorpe T, Dieudonne AS, Hatse S, Lambrechts D, Andrulis IL, Bogdanova N, Antonenkova N, Rogov JI, Prokofieva D, Bermisheva M, Khusnutdinova E, van Asperen CJ, Tollenaar RA, Hooning MJ, Devilee P, Margolin S, Lindblom A, Milne RL, Arias JI, Zamora MP, Benitez J, Severi G, Baglietto L, Giles GG, Spurdle AB, Beesley J, Chen X, Holland H, Healey S, Wang-Gohrke S, Chang-Claude J, Mannermaa A, Kosma VM, Kauppinen J, Kataja V, Agnarsson BA, Caligo MA, Godwin AK, Nevanlinna H, Heikkinen T, Fredericksen Z, Lindor N, Nathanson KL, Domchek SM, Loman N, Karlsson P, Stenmark Askmalm M, Melin B, von Wachenfeldt A, Hogervorst FB, Verheus M, Rookus MA, Seynaeve C, Oldenburg RA, Ligtenberg MJ, Ausems MG, Aalfs CM, Gille HJ, Wijnen JT, Gomez Garcia EB, Peock S, Cook M, Oliver CT, Frost D, Luccarini C, Pichert G, Davidson R, Chu C, Eccles D, Ong KR, Cook J, Douglas F, Hodgson S, Evans DG, Eeles R, Gold B, Pharoah PD, Offit K, Chenevix-Trench G, Easton DF (2012) Breast cancer risk and 6q22.33: combined results from Breast Cancer Association Consortium and Consortium of Investigators on Modifiers of BRCA1/2. PloS one 7(6):e35706. doi:10.1371/journal.pone.0035706
Serrano-Fernandez P, Debniak T, Gorski B, Bogdanova N, Dork T, Cybulski C, Huzarski T, Byrski T, Gronwald J, Wokolorczyk D, Narod SA, Lubinski J (2009) Synergistic interaction of variants in CHEK2 and BRCA2 on breast cancer risk. Breast Cancer Res Treat 117(1):161–165. doi:10.1007/s10549-008-0249-1
Meeks HD, Song H, Michailidou K, Bolla MK, Dennis J, Wang Q, Barrowdale D, Frost D, McGuffog L, Ellis S, Feng B, Buys SS, Hopper JL, Southey MC, Tesoriero A, James PA, Bruinsma F, Campbell IG, Broeks A, Schmidt MK, Hogervorst FB, Beckman MW, Fasching PA, Fletcher O, Johnson N, Sawyer EJ, Riboli E, Banerjee S, Menon U, Tomlinson I, Burwinkel B, Hamann U, Marme F, Rudolph A, Janavicius R, Tihomirova L, Tung N, Garber J, Cramer D, Terry KL, Poole EM, Tworoger SS, Dorfling CM, van Rensburg EJ, Godwin AK, Guenel P, Truong T, Stoppa-Lyonnet D, Damiola F, Mazoyer S, Sinilnikova OM, Isaacs C, Maugard C, Bojesen SE, Flyger H, Gerdes AM, Hansen TV, Jensen A, Kjaer SK, Hogdall C, Hogdall E, Pedersen IS, Thomassen M, Benitez J, Gonzalez-Neira A, Osorio A, Hoya Mde L, Segura PP, Diez O, Lazaro C, Brunet J, Anton-Culver H, Eunjung L, John EM, Neuhausen SL, Ding YC, Castillo D, Weitzel JN, Ganz PA, Nussbaum RL, Chan SB, Karlan BY, Lester J, Wu A, Gayther S, Ramus SJ, Sieh W, Whittermore AS, Monteiro AN, Phelan CM, Terry MB, Piedmonte M, Offit K, Robson M, Levine D, Moysich KB, Cannioto R, Olson SH, Daly MB, Nathanson KL, Domchek SM, Lu KH, Liang D, Hildebrant MA, Ness R, Modugno F, Pearce L, Goodman MT, Thompson PJ, Brenner H, Butterbach K, Meindl A, Hahnen E, Wappenschmidt B, Brauch H, Bruning T, Blomqvist C, Khan S, Nevanlinna H, Pelttari LM, Aittomaki K, Butzow R, Bogdanova NV, Dork T, Lindblom A, Margolin S, Rantala J, Kosma VM, Mannermaa A, Lambrechts D, Neven P, Claes KB, Maerken TV, Chang-Claude J, Flesch-Janys D, Heitz F, Varon-Mateeva R, Peterlongo P, Radice P, Viel A, Barile M, Peissel B, Manoukian S, Montagna M, Oliani C, Peixoto A, Teixeira MR, Collavoli A, Hallberg E, Olson JE, Goode EL, Hart SN, Shimelis H, Cunningham JM, Giles GG, Milne RL, Healey S, Tucker K, Haiman CA, Henderson BE, Goldberg MS, Tischkowitz M, Simard J, Soucy P, Eccles DM, Le N, Borresen-Dale AL, Kristensen V, Salvesen HB, Bjorge L, Bandera EV, Risch H, Zheng W, Beeghly-Fadiel A, Cai H, Pylkas K, Tollenaar RA, Ouweland AM, Andrulis IL, Knight JA, Narod S, Devilee P, Winqvist R, Figueroa J, Greene MH, Mai PL, Loud JT, Garcia-Closas M, Schoemaker MJ, Czene K, Darabi H, McNeish I, Siddiquil N, Glasspool R, Kwong A, Park SK, Teo SH, Yoon SY, Matsuo K, Hosono S, Woo YL, Gao YT, Foretova L, Singer CF, Rappaport-Feurhauser C, Friedman E, Laitman Y, Rennert G, Imyanitov EN, Hulick PJ, Olopade OI, Senter L, Olah E, Doherty JA, Schildkraut J, Koppert LB, Kiemeney LA, Massuger LF, Cook LS, Pejovic T, Li J, Borg A, Ofverholm A, Rossing MA, Wentzensen N, Henriksson K, Cox A, Cross SS, Pasini BJ, Shah M, Kabisch M, Torres D, Jakubowska A, Lubinski J, Gronwald J, Agnarsson BA, Kupryjanczyk J, Moes-Sosnowska J, Fostira F, Konstantopoulou I, Slager S, Jones M, Antoniou AC, Berchuck A, Swerdlow A, Chenevix-Trench G, Dunning AM, Pharoah PD, Hall P, Easton DF, Couch FJ, Spurdle AB, Goldgar DE (2016) BRCA2 polymorphic stop codon K3326X and the risk of breast, prostate, and ovarian cancers. J Natl Cancer Inst. doi:10.1093/jnci/djv315
Thompson ER, Gorringe KL, Rowley SM, Li N, McInerny S, Wong-Brown MW, Devereux L, Li J, Trainer AH, Mitchell G, Scott RJ, James PA, Campbell IG (2015) Reevaluation of the BRCA2 truncating allele c.9976 A > T (p.Lys3326Ter) in a familial breast cancer context. Sci Rep 5:14800. doi:10.1038/srep14800
Healey CS, Dunning AM, Teare MD, Chase D, Parker L, Burn J, Chang-Claude J, Mannermaa A, Kataja V, Huntsman DG, Pharoah PD, Luben RN, Easton DF, Ponder BA (2000) A common variant in BRCA2 is associated with both breast cancer risk and prenatal viability. Nat Genet 26(3):362–364. doi:10.1038/81691
Qiu LX, Yao L, Xue K, Zhang J, Mao C, Chen B, Zhan P, Yuan H, Hu XC (2010) BRCA2 N372H polymorphism and breast cancer susceptibility: a meta-analysis involving 44,903 subjects. Breast Cancer Res Treat 123(2):487–490. doi:10.1007/s10549-010-0767-5
Su L, Wang J, Tao Y, Shao X, Ding Y, Cheng X, Zhu Y (2015) BRCA2 N372H polymorphism and risk of epithelial ovarian cancer: an updated meta-analysis with 2344 cases and 9672 controls. Medicine (Baltimore) 94(42):e1695. doi:10.1097/md.0000000000001695
Xue WQ, He YQ, Zhu JH, Ma JQ, He J, Jia WH (2014) Association of BRCA2 N372H polymorphism with cancer susceptibility: a comprehensive review and meta-analysis. Sci Rep 4:6791. doi:10.1038/srep06791
Cox DG, Simard J, Sinnett D, Hamdi Y, Soucy P, Ouimet M, Barjhoux L, Verny-Pierre C, McGuffog L, Healey S, Szabo C, Greene MH, Mai PL, Andrulis IL, Thomassen M, Gerdes AM, Caligo MA, Friedman E, Laitman Y, Kaufman B, Paluch SS, Borg A, Karlsson P, Askmalm MS, Bustinza GB, Nathanson KL, Domchek SM, Rebbeck TR, Benitez J, Hamann U, Rookus MA, van den Ouweland AM, Ausems MG, Aalfs CM, van Asperen CJ, Devilee P, Gille HJ, Peock S, Frost D, Evans DG, Eeles R, Izatt L, Adlard J, Paterson J, Eason J, Godwin AK, Remon MA, Moncoutier V, Gauthier-Villars M, Lasset C, Giraud S, Hardouin A, Berthet P, Sobol H, Eisinger F, Bressac de Paillerets B, Caron O, Delnatte C, Goldgar D, Miron A, Ozcelik H, Buys S, Southey MC, Terry MB, Singer CF, Dressler AC, Tea MK, Hansen TV, Johannsson O, Piedmonte M, Rodriguez GC, Basil JB, Blank S, Toland AE, Montagna M, Isaacs C, Blanco I, Gayther SA, Moysich KB, Schmutzler RK, Wappenschmidt B, Engel C, Meindl A, Ditsch N, Arnold N, Niederacher D, Sutter C, Gadzicki D, Fiebig B, Caldes T, Laframboise R, Nevanlinna H, Chen X, Beesley J, Spurdle AB, Neuhausen SL, Ding YC, Couch FJ, Wang X, Peterlongo P, Manoukian S, Bernard L, Radice P, Easton DF, Chenevix-Trench G, Antoniou AC, Stoppa-Lyonnet D, Mazoyer S, Sinilnikova OM (2011) Common variants of the BRCA1 wild-type allele modify the risk of breast cancer in BRCA1 mutation carriers. Hum Mol Genet 20(23):4732–4747. doi:10.1093/hmg/ddr388
Dunning AM, Chiano M, Smith NR, Dearden J, Gore M, Oakes S, Wilson C, Stratton M, Peto J, Easton D, Clayton D, Ponder BA (1997) Common BRCA1 variants and susceptibility to breast and ovarian cancer in the general population. Hum Mol Genet 6(2):285–289
Freedman ML, Penney KL, Stram DO, Le Marchand L, Hirschhorn JN, Kolonel LN, Altshuler D, Henderson BE, Haiman CA (2004) Common variation in BRCA2 and breast cancer risk: a haplotype-based analysis in the Multiethnic Cohort. Hum Mol Genet 13(20):2431–2441. doi:10.1093/hmg/ddh270
Neuhausen SL, Mazoyer S, Friedman L, Stratton M, Offit K, Caligo A, Tomlinson G, Cannon-Albright L, Bishop T, Kelsell D, Solomon E, Weber B, Couch F, Struewing J, Tonin P, Durocher F, Narod S, Skolnick MH, Lenoir G, Serova O, Ponder B, Stoppa-Lyonnet D, Easton D, King MC, Goldgar DE (1996) Haplotype and phenotype analysis of six recurrent BRCA1 mutations in 61 families: results of an international study. Am J Hum Genet 58(2):271–280
Pilato B, Martinucci M, Danza K, Pinto R, Petriella D, Lacalamita R, Bruno M, Lambo R, D’Amico C, Paradiso A, Tommasi S (2011) Mutations and polymorphic BRCA variants transmission in breast cancer familial members. Breast Cancer Res Treat 125(3):651–657. doi:10.1007/s10549-010-0861-8
de Vree PJ, de Wit E, Yilmaz M, van de Heijning M, Klous P, Verstegen MJ, Wan Y, Teunissen H, Krijger PH, Geeven G, Eijk PP, Sie D, Ylstra B, Hulsman LO, van Dooren MF, van Zutven LJ, van den Ouweland A, Verbeek S, van Dijk KW, Cornelissen M, Das AT, Berkhout B, Sikkema-Raddatz B, van den Berg E, van der Vlies P, Weening D, den Dunnen JT, Matusiak M, Lamkanfi M, Ligtenberg MJ, ter Brugge P, Jonkers J, Foekens JA, Martens JW, van der Luijt R, van Amstel HK, van Min M, Splinter E, de Laat W (2014) Targeted sequencing by proximity ligation for comprehensive variant detection and local haplotyping. Nat Biotechnol 32(10):1019–1025. doi:10.1038/nbt.2959
Acknowledgements
This work would not have been possible without the inspiration, concept and continued effort of Rita Schmutzler and Alfons Meindl and all collaborators of GC-HBOC. We thank the UCV-task force of GC-HBOC for classification of the genetic variants. Special thanks go to Wolfgang Distler for persistent support and encouragement. We thank the German Cancer Aid for supporting of GC-HBOC with Grant No. 110837.
Authors contributions
KK: conception of the work, acquisition, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, final approval of the version to be published. CM: acquisition and interpretation of data, critical revision of the manuscript for important intellectual content, final approval of the version to be published. CES: analysis and interpretation of data, critical revision of the manuscript for important intellectual content, final approval of publication. DK: analysis and interpretation of the data, final approval of publication. NA, PW, AR: interpretation of data, critical revision of the manuscript for important intellectual content, final approval of publication. All other authors: acquisition of data, critical revision of the manuscript for important intellectual content, final approval of publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
German Cancer Aid Grant No 110837 for GC-HBOC.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (No. EK 162072007) and with the 1064 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
C. Meisel and C. E. Sadowski contributed equally to this work.
Rights and permissions
About this article
Cite this article
Meisel, C., Sadowski, C.E., Kohlstedt, D. et al. Spectrum of genetic variants of BRCA1 and BRCA2 in a German single center study. Arch Gynecol Obstet 295, 1227–1238 (2017). https://doi.org/10.1007/s00404-017-4330-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4330-z