Abstract
Objective
Primary neuroendocrine tumors in the ovary are rare. These tumors arise from the neuroendocrine cell system of ovarian stroma and surface epithelium, and may also arise from teratoma. We present four primary ovarian neuroendocrine tumors and compare clinicopathologic findings based on tumor histogenesis and site of origin.
Design
Four primary ovarian neuroendocrine tumors were identified from our 10-year departmental archives. H&E slides and immunostains were reviewed and the diagnoses were confirmed. Clinical history, imaging studies, and follow-up data were obtained from medical records.
Results
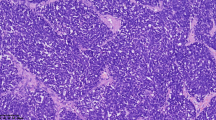
Patients' ages ranged from 26 to 63. All patients presented with abdominal discomfort and unilateral or bilateral ovarian masses. MRI and CT scans from cases 1 and 2 revealed a solid ovarian mass with no extra-ovarian extension. In case 1, the patient also had a cystic mass in the opposite ovary and an elevated urine 5-HIAA. Microscopically, case 1 revealed a well-differentiated carcinoid tumor with no surface epithelial involvement, and a mature teratoma in the contralateral ovary. Case 2 revealed a stromal carcinoid within the ovarian parenchyma. Imaging studies from cases 3 and 4 showed large complex masses with peritoneal implants and ascites. In both cases 3 and 4, tumor grossly involved both ovarian parenchyma and surface epithelium with multiple pelvic implants. In addition, liver metastases were present in case 4. Microscopically, these tumors were poorly differentiated carcinoma with neuroendocrine differentiation. Histologic sections revealed extensive necrosis, and both cases showed positivity for neuroendocrine markers.
Conclusions
Primary neuroendocrine tumors in the ovary are rare and consist of a group of heterogeneous malignancies that express similar immunohistochemical markers. Primary neuroendocrine tumors that are limited to the ovarian parenchyma often arise from ovarian stroma and teratoma, and are carcinoid tumors with a good prognosis. Neuroendocrine tumors that arise from surface epithelium or dedifferentiate from de novo carcinoma often involve both ovarian stroma and surface epithelium and clinically present as aggressive malignancies with poor prognoses.



Similar content being viewed by others
References
Gardner GJ, Reidy-Lagunes D, Gehrig PA (2011) Neuroendocrine tumors of the gynecologic tract: a Society of Gynecologic Oncology (SGO) clinical document. Gynecol Oncol 122(1):190–198. doi:10.1016/j.ygyno.2011.04.011
Rouzbahman M, Clarke B (2013) Neuroendocrine tumors of the gynecologic tract: select topics. Semin Diagn Pathol 30(3):224–233. doi:10.1053/j.semdp.2013.06.007
Robboy SJ, Norris HJ, Scully RE (1975) Insular carcinoid primary in the ovary. A clinicopathologic analysis of 48 cases. Cancer 36(2):404–418
Dundr P, Fischerova D, Povysil C, Cibula D (2008) Primary pure large-cell neuroendocrine carcinoma of the ovary. Pathol Res Pract 204(2):133–137. doi:10.1016/j.prp.2007.09.004
Veras E, Deavers MT, Silva EG, Malpica A (2007) Ovarian nonsmall cell neuroendocrine carcinoma: a clinicopathologic and immunohistochemical study of 11 cases. Am J Surg Pathol 31(5):774–782. doi:10.1097/01.pas.0000213422.53750.d1
Roth LM, Goheen MP, Broshears JR (2012) Malignant Brenner tumor of the ovary with transformation to trabecular carcinoid: an immunocytochemical and electron microscopic study. Int J Gynecol Pathol 31(1):91–97. doi:10.1097/PGP.0b013e3182230e10
Mhawech-Fauceglia P, Odunsi K, Dim D, Nowak N, Lele S, Cheney RT, Pejovic T (2008) Array-comparative genomic hybridization analysis of primary endometrial and ovarian high-grade neuroendocrine carcinoma associated with adenocarcinoma: mystery resolved? Int J Gynecol Pathol 27(4):539–546. doi:10.1097/PGP.0b013e31816bcda4
Eichhorn JH, Lawrence WD, Young RH, Scully RE (1996) Ovarian neuroendocrine carcinomas of non-small-cell type associated with surface epithelial adenocarcinomas. A study of five cases and review of the literature. Int J Gynecol Pathol 15(4):303–314
Modlin IM, Lye KD, Kidd M (2003) A 5-decade analysis of 13,715 carcinoid tumors. Cancer 97(4):934–959. doi:10.1002/cncr.11105
Meinhold-Heerlein I, Hauptmann S (2014) The heterogeneity of ovarian cancer. Arch Gynecol Obstet 289(2):237–239. doi:10.1007/s00404-013-3114-3
Talerman A (1984) Carcinoid tumors of the ovary. J Cancer Res Clin Oncol 107(2):125–135
Diaz-Montes TP, Rosenthal LE, Bristow RE, Grumbine FC (2006) Primary insular carcinoid of the ovary. Gynecol Oncol 101(1):175–178. doi:10.1016/j.ygyno.2005.10.015
Davis KP, Hartmann LK, Keeney GL, Shapiro H (1996) Primary ovarian carcinoid tumors. Gynecol Oncol 61(2):259–265. doi:10.1006/gyno.1996.0136
Kurabayashi T, Minamikawa T, Nishijima S, Tsuneki I, Tamura M, Yanase T, Hashidate H, Shibuya H, Motoyama T (2010) Primary strumal carcinoid tumor of the ovary with multiple bone and breast metastases. J Obstet Gynaecol Res 36(3):567–571. doi:10.1111/j.1447-0756.2010.01231.x
Collins RJ, Cheung A, Ngan HY, Wong LC, Chan SY, Ma HK (1991) Primary mixed neuroendocrine and mucinous carcinoma of the ovary. Arch Gynecol Obstet 248(3):139–143
Alenghat E, Okagaki T, Talerman A (1986) Primary mucinous carcinoid tumor of the ovary. Cancer 58(3):777–783
Buis CC, van Doorn HC, Dinjens WN, Ewing PC (2010) Mucinous carcinoid of the ovary: report of a case with metastasis in the contralateral ovary after 10 years. Rare Tumors 2(3):e39. doi:10.4081/rt.2010.e39
Baker PM, Oliva E, Young RH, Talerman A, Scully RE (2001) Ovarian mucinous carcinoids including some with a carcinomatous component: a report of 17 cases. Am J Surg Pathol 25(5):557–568
Strosberg J, Nasir A, Cragun J, Gardner N, Kvols L (2007) Metastatic carcinoid tumor to the ovary: a clinicopathologic analysis of seventeen cases. Gynecol Oncol 106(1):65–68. doi:10.1016/j.ygyno.2007.02.034
Rabban JT, Lerwill MF, McCluggage WG, Grenert JP, Zaloudek CJ (2009) Primary ovarian carcinoid tumors may express CDX-2: a potential pitfall in distinction from metastatic intestinal carcinoid tumors involving the ovary. Int J Gynecol Pathol 28(1):41–48. doi:10.1097/PGP.0b013e31817a8f51
Lin X, Saad RS, Luckasevic TM, Silverman JF, Liu Y (2007) Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol 15(4):407–414. doi:10.1097/01.pai.0000210416.53493.0f
Desouki MM, Lioyd J, Xu H, Cao D, Barner R, Zhao C (2013) CDX2 may be a useful marker to distinguish primary ovarian carcinoid from gastrointestinal metastatic carcinoids to the ovary. Hum Pathol 44(11):2536–2541. doi:10.1016/j.humpath.2013.06.014
McCluggage WG, McKenna M, McBride HA (2007) CD56 is a sensitive and diagnostically useful immunohistochemical marker of ovarian sex cord-stromal tumors. Int J Gynecol Pathol 26(3):322–327. doi:10.1097/01.pgp.0000236947.59463.87
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Vora, M., Lacour, R.A., Black, D.R. et al. Neuroendocrine tumors in the ovary: histogenesis, pathologic differentiation, and clinical presentation. Arch Gynecol Obstet 293, 659–665 (2016). https://doi.org/10.1007/s00404-015-3865-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3865-0