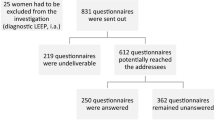
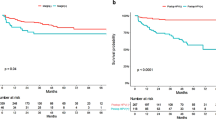
Abstract
Objective
This study aimed at assessing the association of the relative risk (RR) of adverse pregnancy outcomes with previous treatment of loop electrosurgical excision procedure (LEEP) for the management of cervical intraepithelial neoplasia (CIN).
Methods
Data sources were from MEDLINE, EMBASE, and SCI citation tracking. Selection criteria: The eligible studies had data on pregnancy outcomes of women with or without previous treatment for CIN. Considered outcomes were severe preterm delivery (<34/32 weeks), extreme preterm delivery (<28 weeks), low birth weight (<2,500 g), stillbirth, preterm spontaneous rupture of membranes, perinatal mortality, and neonatal mortality and induction.
Results
36,954 cases and 1,794,174 controls in 4 prospective cohort and 22 retrospective studies were included in this meta-analysis. LEEP was associated with a higher risk of severe preterm delivery (<32 weeks, relative risk 1.98, 95 % CI [1.31, 2.98] 159/11,337 vs. 7,830/860,883), extreme preterm delivery (<28 weeks, RR, 2.33, 95 % CI [1.84, 2.94] 97/9,611 vs. 1,559/618,332), preterm premature rupture of the membranes (RR, 1.88, 95 % CI [1.54, 2.29] 126/2,837 vs. 7,899/313,094), and low birth weight (<2,500 g, RR, 2.48, 95 % CI [1.75, 3.51] 110/1,451 vs. 55/1,742). A cervical length of less than 3 cm was significantly increased in LEEP as compared with that of control group (RR, 4.88, 95 % CI [1.56, 15.25]), but increasing LEEP volume or depth was not associated with an increased rate of preterm birth <37 weeks. And LEEP was not associated with a significantly increased risk of perinatal mortality, cesarean section, stillbirth mortality, neonatal mortality, induction, and neonatal intensive care unit admission.
Conclusions
LEEP is associated with an increased risk of subsequent preterm delivery (<32/34, <28 weeks) and other serious pregnancy outcomes. But increasing LEEP volume or depth is not associated with an increased rate of preterm birth.








Similar content being viewed by others
References
Jones BA, Davey DD (2000) Quality management in gynecologic cytology using interlaboratory comparison. Arch Pathol Lab Med 124(5):672–681
Koutsky L (1997) Epidemiology of genital human papillomavirus infection. Am J Med 102(5S1):3–8
Paraskevaidis E, Kitchener HC, Miller ID, Mann E, Jandial L, Fisher PM (1992) A population-based study of microinvasive disease of the cervix: a colposcopic and cytologic analysis. Gynecol Oncol 45(1):9–12
Herbert A (2000) Cervical screening in England and Wales: its effect has been underestimated. Cytopathology 11(6):471–479
Perlman SE, Lubianca JN, Kahn JA (2003) Characteristics of a group of adolescents undergoing loop electrical excision procedure (LEEP). J Pediatr Adolesc Gynecol 16(1):15–20
Keijser KGG, Kenemans P, Vanderzanden P, Schijf CPT, Vooijs GP, Rolland R (1992) Diathermy loop excision in the management of cervical intraepithelial neoplasia: diagnosis and treatment in one procedure. Am J Obst Gynecol 166(4):1281–1287
Kitchener H, Cruickshank M, Farmery E (1995) The 1993 British society for colposcopy and cervical pathology/National coordinating network United Kingdom colposcopy survey. BJOG 102(7):549–552
Crane JMG (2003) Pregnancy outcome after loop electrosurgical excision procedure: a systematic review. Obstet Gynecol 102(5):1058–1062. doi:10.1016/s0029-7844(03)00741-5
Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin-Hirsch P, Prendiville W, Paraskevaidis E (2008) Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. Br Med J 337(7673). doi:10.1136/bmj.a1284
Jakobsson M, Gissler M, Paavonen J, Tapper A-M (2009) Loop electrosurgical excision procedure and the risk for preterm birth. Obst Gynecol 114(3):504–510
Gentry DJ, Baggish MS, Brady K, Walsh PM, Hungler MS (2000) The effects of loop excision of the transformation zone on cervical length: Implications for pregnancy. Am J Obst Gynecol 182(3):516–520. doi:10.1067/mob.2000.104209
Ricciotti HA, Burke L, Kobelin M, Slomovic B, Ludmir J (1995) Ultrasound evaluation of cervical shortening after loop excision of the transformation zone (letz). Intern J Gynecol Obst 50(2):175–178. doi:10.1016/0020-7292(95)02432-c
Noehr B, Jensen A, Frederiksen K, Tabor A, Kjaer SK (2009) Loop electrosurgical excision of the cervix and subsequent risk for spontaneous preterm delivery: a population-based study of singleton deliveries during a 9-year period. Am J Obst Gynecol 201(1):33e1–33e6
Cruickshank ME, Flannelly G, Campbell DM, Kitchener HC (1995) Fertility and pregnancy outcome following large loop excision of the cervical transformation zone. Br J Obst Gynecol 102(6):467–470. doi:10.1111/j.1471-0528.1995.tb11319.x
Bruinsma F, Lumley J, Tan J, Quinn M (2007) Precancerous changes in the cervix and risk of subsequent preterm birth. BJOG 114(1):70–80
Samson SLA, Bentley JR, Fahey TJ, McKay DJ, Gill GH (2005) The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obst Gynecol 105(2):325–332
Fischer RL, Sveinbjornsson G, Hansen C (2010) Cervical sonography in pregnant women with a prior cone biopsy or loop electrosurgical excision procedure. Ultrasound Obst Gynecol 36(5):613–617. doi:10.1002/uog.7682
Stout M, Tuuli M, Cahill A, Odibo A, Stamilio D, Macones G (2012) Do women with a history of LEEP and active vaginal infections during pregnancy have an increased risk for preterm birth? Am J Obstet Gynecol 206(1):S157
Poon LCY, Savvas M, Zamblera D, Skyfta E, Nicolaides KH (2012) Large loop excision of transformation zone and cervical length in the prediction of spontaneous preterm delivery. BJOG 119(6):692–698
Macones GA, Cahill A, Stamilio D, Roehl K, Odibo A (2012) Pregnancy after LEEP: results of a multicenter study. Am J Obstet Gynecol 206(1):S3–S4
Simoens C, Goffin F, Simon P, Barlow P, Antoine J, Arbyn JM, Arbyn M (2012) Adverse obstetrical outcomes after treatment of precancerous cervical lesions: a Belgian multicentre study. BJOG 119(10):1247–1255. doi:10.1111/j.1471-0528.2012.03429.x
Lima AF, Francisco C, Julio C, Paula T, Vitorino A, Borrego J (2011) Obstetric outcomes after treatment for cervical intraepithelial neoplasia: six years of experience. J Low Genit Tract Dis 15(4):276–279
Andia D, Mozo de Rosales F, Villasante A, Rivero B, Diez J, Perez C (2011) Pregnancy outcome in patients treated with cervical conization for cervical intraepithelial neoplasia. Intern J Gynecol Obst 112(3):225–228. doi:10.1016/j.ijgo.2010.10.015
Ortoft G, Henriksen TB, Hansen ES, Petersen LK (2010) After conisation of the cervix, the perinatal mortality as a result of preterm delivery increases in subsequent pregnancy. BJOG 117(3):258–267. doi:10.1111/j.1471-0528.2009.02438.x
Crane JMG, Delaney T, Hutchens D (2006) Transvaginal ultrasonography in the prediction of preterm birth after treatment for cervical intraepithelial neoplasia. Obst Gynecol 107(1):37–44. doi:10.1097/01.aog.0000192169.44775.76
Acharya G, Kjeldberg I, Hansen SM, Sorheim N, Jacobsen BK, Maltau JM (2005) Pregnancy outcome after loop electrosurgical excision procedure for the management of cervical intraepithelial neoplasia. Arch Gynecol Obstet 272(2):109–112. doi:10.1007/s00404-005-0727-1
Sadler L, Saftlas A, Wang WQ, Exeter M, Whittaker J, McCowan L (2004) Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA 291(17):2100–2106. doi:10.1001/jama.291.17.2100
Werner CL, Lo JY, Heffernan T, Griffith WF, McIntire DD, Leveno KJ (2010) Loop electrosurgical excision procedure and risk of preterm birth. Obstet Gynecol 115(3):605–608
Braet P, Peel J, Fenton D (1994) A case controlled study of the outcome of pregnancy following loop diathermy excision of the transformation zone. J Obst Gynecol 14(2):79–82
Blomfield PI, Buxton J, Dunn J, Luesley DM (1993) Pregnancy outcome after large loop excision of the cervical transformation zone. Am J Obstet Gynecol 169(3):620–625
Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E (2006) Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 367(9509):489–498. doi:10.1016/s0140-6736(06)68181-6
Macones GA, Cahill A, Stamilio D, Roehl K, Odibo A (2012) Does LEEP specimen size influence the risk of preterm birth? Am J Obstet Gynecol 206(1):S218
Khalid S, Dimitriou E, Conroy R, Paraskevaidis E, Kyrgiou M, Harrity C, Arbyn M, Prendiville W (2012) The thickness and volume of LLETZ specimens can predict the relative risk of pregnancy-related morbidity. BJOG 119(6):685–691
Jakobsson M, Gissler M, Sainio S, Paavonen J, Tapper A-M (2007) Preterm delivery after surgical treatment for cervical intraepithelial neoplasia. Obstet Gynecol 109(2):309–313. doi:10.1097/01.aog.0000253239.87040.23
Beta J, Akolekar R, Ventura W, Syngelaki A, Nicolaides KH (2011) Prediction of spontaneous preterm delivery from maternal factors, obstetric history and placental perfusion and function at 11–13 weeks. Prenat Diagn 31(1):75–83
Balchin I, Steer PJ (2007) Race, prematurity and immaturity. Early Hum Dev 83(12):749–754
Crane JMG, Delaney T, Hutchens D (2006) Transvaginal ultrasonography in the prediction of preterm birth after treatment for cervical intraepithelial neoplasia. Obstet Gynecol 107(1):37–44
Parikh R, Horne H, Feinstein S, Anasti J (2008) Cervical length screening in patients who have undergone loop electrosurgical excision procedure. J Reprod Med 53(12):909
Sadler L, Saftlas A, Wang W, Exeter M, Whittaker J, McCowan L (2004) Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA 291(17):2100–2106
Strander B, Andersson-Ellström A, Milsom ISparén P (2007) Long term risk of invasive cancer after treatment for cervical intraepithelial neoplasia grade 3: population based cohort study. BMJ 335(7629):1077
Soutter WP, Sasieni P, Panoskaltsis T (2005) Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int J Cancer 118(8):2048–2055
Kalliala I, Anttila A, Pukkala E, Nieminen P (2005) Risk of cervical and other cancers after treatment of cervical intraepithelial neoplasia: retrospective cohort study. BMJ 331(7526):1183–1185
Bruinsma F, Lumley J, Tan J, Quinn M (2006) Precancerous changes in the cervix and risk of subsequent preterm birth. BJOG 114(1):70–80
Paraskevaidis E, Koliopoulos G, Lolis E, Papanikou E, Malamou-Mitsi V, Agnantis NJ (2002) Delivery outcomes following loop electrosurgical excision procedure for microinvasive (FIGO stage 1A1) cervical cancer. Gynecol Oncol 86(1):10–13. doi:10.1006/gyno.2002.6650
Tan L, Pepra E, Haloob RK (2004) The outcome of pregnancy after large loop excision of the transformation zone of the cervix. J Obst Gynecol 24(1):25–27
Noehr B, Jensen A, Frederiksen K, Kjaer A, Kjaer SK (2009) Depth of cervical cone removed by loop electrosurgical excision procedure and subsequent risk of spontaneous preterm delivery. Obstet Gynecol 114(6):1232–1238
Paavonen J, Heinonen A, Gissler M, Jakobsson AM, Jakobsson M (2011) Leep conisation and the risk for preterm birth: new health registry based data from Finland. Sex Trans Inf 87:A357. doi:10.1136/sextrans-2011-050119.19
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
G. Jin and Z. LanLan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jin, G., LanLan, Z., Li, C. et al. Pregnancy outcome following loop electrosurgical excision procedure (LEEP) a systematic review and meta-analysis. Arch Gynecol Obstet 289, 85–99 (2014). https://doi.org/10.1007/s00404-013-2955-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-2955-0
Keywords
- Cervical intraepithelial neoplasia (CIN)
- Cesarean section (CS)
- Cervical volume
- Large loop excision of the transformation zone (LLETZ)
- Loop electrosurgical excision procedure (LEEP)
- Lower birth weight (LBW)
- Preterm delivery (PD)
- Perinatal mortality (PM)
- Pregnancy outcome
- Preterm spontaneous rupture of membranes (PPROM)
- Stillbirth mortality