Abstract
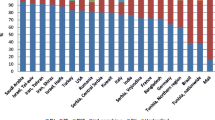
Autoimmune bullous diseases are rare, organ-specific, a group of blistering disease of skin and mucous membranes. Recent studies suggest that the frequency of the autoimmune bullous diseases has been increasing. Pemphigus vulgaris and bullous pemphigoid are the most frequently reported autoimmune bullous diseases. High incidence of autoimmune bullous diseases in some ethnic groups such as pemphigus in Ashkenazi Jewish, or in some regions such as pemphigus foliaceus in Brazil has been shown to be related to genetic and environmental factors, respectively. Pemphigus has been reported more frequently in the female gender. Although it is most frequently diagnosed between the ages 50 and 60 in European countries, in the remaining countries in the world, it is seen between the ages of 30 and 50. Bullous pemphigoid is generally seen above 70 years of age. Although overall incidence is slightly higher in females, after the age of 80 years it is more frequent in males. Both pemphigus vulgaris and bullous pemphigoid has a chronic course with recurrences. Mortality risk of the patients with bullous pemphigoid was found at least 2 times higher and the mortality risk of the patients with pemphigus was found approximately 3 times higher than that of the general population. In this review, the results obtained from the epidemiological studies were analyzed according to geographic regions, and especially epidemiologic features of two prevalent autoimmune bullous diseases, pemphigus and bullous pemphigoid have been discussed.
Similar content being viewed by others
References
Aboobaker J, Morar N, Ramdial PK, Hammond MG (2001) Pemphigus in South Africa. Int J Dermatol 40:115–119
Adam BA (1992) Bullous diseases in Malaysia: epidemiology and natural history. Int J Dermatol 31:42–45
Ahmed AR, Wagner R, Khatri K et al (1991) Major histocompatibility complex haplotypes and class II genes in non-Jewish patients with pemphigus vulgaris. Proc Natl Acad Sci USA 88:5056–5060
Ahmed AR, Yunis EJ, Khatri K et al (1990) Major histocompatibility complex haplotype studies in Ashkenazi Jewish patients with pemphigus vulgaris. Proc Natl Acad Sci USA 87:7658–7662
Akman A, Kacaroglu H, Yilmaz E et al (2008) Periodontal status in patients with pemphigus vulgaris. Oral Dis 14:640–643
Amin MN, Islam AZ (2006) Clinical, histologic and immunologic features of pemphigus in Bangladesh. Int J Dermatol 45:1317–1318
Baican A, Baican C, Chiriac G et al (2010) Pemphigus vulgaris is the most common autoimmune bullous disease in Northwestern Romania. Int J Dermatol 49:768–774
Bastuji-Garin S, Joly P, Lemordant P et al (2011) Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol 131:637–643
Bastuji-Garin S, Joly P, Picard-Dahan C et al (1996) Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol 132:272–276
Bastuji-Garin S, Souissi R, Blum L et al (1995) Comparative epidemiology of pemphigus in Tunisia and France: unusual incidence of pemphigus foliaceus in young Tunisian women. J Invest Dermatol 104:302–305
Benchikhi H, Ghafour S, Disky A et al (2008) Pemphigus: analysis of 262 cases. Int J Dermatol 47:973–975
Bernard P, Vaillant L, Labeille B et al (1995) Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Bullous Diseases French Study Group. Arch Dermatol 131:48–52
Bertram F, Bröcker EB, Zillikens D et al (2009) Prospective analysis of the incidence of autoimmune bullous disorders in Lower Franconia, Germany. J Dtsch Dermatol Ges 7:434–440
Bourdon-Lanoy E, Roujeau JC, Joly P et al (2005) Bullous pemphigoid in young patients: a retrospective study of 74 cases. Ann Dermatol Venereol 132:115–122
Brenner S, Tur E, Shapiro J (2001) Pemphigus vulgaris: environmental factors. Occupational, behavioral, medical, and qualitative food frequency questionnaire. Int J Dermatol 40:562–569
Chams-Davatchi C, Valikhani M, Daneshpazhooh M et al (2005) Pemphigus: analysis of 1209 cases. Int J Dermatol 44:470–476
Colbert RL, Allen DM, Eastwood D et al (2004) Mortality rate of bullous pemphigoid in a US medical center. J Invest Dermatol 122:1091–1095
Cozzani E, Parodi A, Rebora A et al (2001) Gruppo Ligure di Studi in Dermatologia (GLISID). Bullous pemphigoid in Liguria: a 2-year survey. J Eur Acad Dermatol Venereol 15:317–319
Culton DA, Qian Y, Li N et al (2008) Advances in pemphigus and its endemic pemphigus foliaceus (Fogo Selvagem) phenotype: a paradigm of human autoimmunity. J Autoimmun 31:311–324
Daneshpazhooh M, Chams-Davatchi C, Payandemehr P et al (2012) Spectrum of autoimmune bullous diseases in Iran: a 10-year review. Int J Dermatol 51:35–41
Delgado JC, Hameed A, Yunis JJ et al (1997) Pemphigus vulgaris autoantibody response is linked to HLA-DQB1*0503 in Pakistani patients. Hum Immunol 57:110–119
Delgado JC, Turbay D, Yunis EJ et al (1996) A common major histocompatibility complex class II allele HLA-DQB1* 0301 is present in clinical variants of pemphigoid. Proc Natl Acad Sci USA 6(93):8569–8571
Delgado JC, Yunis DE, Bozon MV et al (1996) MHC class II alleles and haplotypes in patients with pemphigus vulgaris from India. Tissue Antigens 48:668–672
Diaz LA, Glamb RW, Silva J Jr (1980) A syndrome of multiple immune autoreactivity. A breakdown in immune regulation. Arch Dermatol 116:77–79
Firooz A, Mazhar A, Ahmed AR (1994) Prevalence of autoimmune diseases in the family members of patients with pemphigus vulgaris. J Am Acad Dermatol 31(3 Pt 1):434–437
Golusin Z, Poljacki M, Jovanovic M et al (2005) Some epidemiological features of pemphigus chronicus in South Vojvodina: a 12-year retrospective study. Int J Dermatol 44:792–793
Gudi VS, White MI, Cruickshank N et al (2005) Annual incidence and mortality of bullous pemphigoid in the Grampian Region of North-east Scotland. Br J Dermatol 153:424–427
Hahn-Ristic K, Rzany B, Amagai M et al (2002) Increased incidence of pemphigus vulgaris in southern Europeans living in Germany compared with native Germans. J Eur Acad Dermatol Venereol 16:68–71
Hietanen J, Salo OP (1982) Pemphigus: an epidemiological study of patients treated in Finnish hospitals between 969 and 1978. Acta Derm Venereol 62:491–496
Huang YH, Kuo CF, Chen YH et al (2012) Incidence, mortality, and causes of death of patients with pemphigus in Taiwan: a Nationwide Population-Based Study. J Invest Dermatol 132:92–97
Huh WK, Tada J, Fujimoto W et al (2001) Thyroid gland tumour, pemphigus foliaceus and myasthenia gravis in the daughter of a woman with myasthenia gravis. Clin Exp Dermatol 26:504–506
Jin P, Shao C, Ye G (1993) Chronic bullous dermatoses in China. Int J Dermatol 32:89–92
Joly P, Baricault S, Sparsa A et al (2012) Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol 132:1998–2004
Jung M, Kippes W, Messer G et al (1999) Increased risk of bullous pemphigoid in male and very old patients: a population-based study on incidence. J Am Acad Dermatol 41:266–268
Kaya Koc C, Sallakci N, Akman-Karakaş A et al (2013) HLA class I and class II antigens in the patients with pemphigus in Southern Turkey. Int J Dermatol 52:53–58
Krain LS, Terasaki PI, Newcomer VD et al (1973) Increased frequency of HLA-A10 in pemphigus vulgaris. Arch Dermatol 108:803–805
Kumar KA (2008) Incidence of pemphigus in Thrissur district, south India. Indian J Dermatol Venereol Leprol 74:349–351
Langan SM, Smeeth L, Hubbard R et al (2008) Bullous pemphigoid and pemphigus vulgaris–incidence and mortality in the UK: population based cohort study. BMJ 337:a180
Lee JJ, Downham TF (2006) Furosemide-induced bullous pemphigoid. Case report and review of literature. J Drugs Dermatol 5:562–564
Leshem YA, Katzenelson V, Yosipovitch G, David M, Mimouni D (2011) Autoimmune diseases in patients with pemphigus and their first-degree relatives. Int J Dermatol 50:827–831
Lindelöf B, Islam N, Eklund G et al (1990) Pemphigoid and cancer. Arch Dermatol 126:66–68
Lo Schiavo A, Ruocco E, Brancaccio G et al (2013) Bullous pemphigoid: etiology, pathogenesis, and inducing factors: Facts and controversies. Clin Dermatol 31:391–399
Mahé A, Flageul B, Cissé I et al (1996) Pemphigus in Mali: a study of 30 cases. Br J Dermatol 134:114–119
Marazza G, Pham HC, Schärer L et al (2009) Incidence of bullous pemphigoid and pemphigus in Switzerland: a 2-year prospective study. Br J Dermatol 161:861–868
Meyer N, Misery L (2010) Geoepidemiologic considerations of auto-immune pemphigus. Autoimmun Rev 9:A379–A382
Micali G, Musumeci ML, Nasca MR (1998) Epidemiologic analysis and clinical course of 84 consecutive cases of pemphigus in eastern Sicily. Int J Dermatol 37:197–200
Michailidou EZ, Belazi MA, Markopoulos AK et al (2007) Epidemiologic survey of pemphigus vulgaris with oral manifestations in northern Greece: retrospective study of 129 patients. Int J Dermatol 46:356–361
Mimouni D, Bar H, Gdalevich M et al (2008) Pemphigus-analysis of epidemiological factors in 155 patients. J Eur Acad Dermatol Venereol 22:1232–1235
Nanda A, Dvorak R, Al-Saeed K et al (2004) Spectrum of autoimmune bullous diseases in Kuwait. Int J Dermatol 43:876–881
Okazaki A, Miyagawa S, Yamashina Y, Kitamura W, Shirai T (2000) Polymorphisms of HLA-DR and -DQ genes in Japanese patients with bullous pemphigoid. J Dermatol 27:149–156
Ong E, Goldacre R, Hoang U et al (2014) Associations between bullous pemphigoid and primary malignant cancers: an English national record linkage study, 1999–2011. Arch Dermatol Res 306:75–80
Park MS, Terasaki PI, Ahmed AR et al (1979) HLA-DRW4 in 91% of pemphigus vulgaris patients. Lancet 2:441–442
Pisanti S, Sharav Y, Kaufman E et al (1974) Pemphigus vulgaris: incidence in Jews of different ethnic groups, according to age, sex, and initial lesion. Oral Surg Oral Med Oral Pathol 38:382–387
Roujeau JC, Lok C, Bastuji-Garin S et al (1998) High risk of death in elderly patients with extensive bullous pemphigoid. Arch Dermatol 134:465–469
Ruocco V, Ruocco E, Lo Schiavo A et al (2013) Pemphigus: etiology, pathogenesis, and inducing or triggering factors: facts and controversies. Clin Dermatol 31:374–381
Salmanpour R, Shahkar H, Namazi MR et al (2006) Epidemiology of pemphigus in south-western Iran: a 10-year retrospective study (1991–2000). Int J Dermatol 45:103–105
Serwin AB, Bokiniec E, Piascik M et al (2007) Epidemiological and clinical analysis of pemphigoid patients in northeastern Poland in 2000-2005. Med Sci Monit 13:360–364
Simon DG, Krutchkoff D, Kaslow RA et al (1980) Pemphigus in Hartford County, Connecticut, from 1972 to 1977. Arch Dermatol 116:1035–1037
Sullivan TP, Elgart GW, Kirsner RS (2002) Pemphigus and smoking. Int J Dermatol 41:528–530
Tallab T, Joharji H, Bahamdan K et al (2001) The incidence of pemphigus in the southern region of Saudi Arabia. Int J Dermatol 40:570–572
Tsankov N, Vassileva S, Kamarashev J et al (2000) Epidemiology of pemphigus in Sofia, Bulgaria. A 16-year retrospective study (1980–1995). Int J Dermatol 39:104–108
Uzun S, Durdu M, Akman A et al (2006) Pemphigus in the mediterranean region of Turkey: a study of 148 cases. Int J Dermatol 45:523–528
Vega-Memije ME, Saez de Ocariz-Gutierrez MM, Corte-Franco R et al (2001) Analysis of HLA-DR in Mexican patients with pemphigus. Gac Med Mex 137:535–540
Venning VA, Wojnarowska F (1995) Induced bullous pemphigoid. Br J Dermatol 132:831–832
V’lckova-Laskoska MT, Laskoski DS, Kamberova S et al (2007) Epidemiology of pemphigus in Macedonia: a 15-year retrospective study (1990–2004). Int J Dermatol 46:253–258
Waisbourd-Zinman O, Ben-Amitai D, Cohen AD et al (2008) Bullous pemphigoid in infancy: clinical and epidemiologic characteristics. J Am Acad Dermatol 58:41–48
Wong SN, Chua SH (2002) Spectrum of subepidermal immunobullous disorders seen at the National Skin Centre, Singapore: a 2-year review. Br J Dermatol 147:476–480
Zakka LR, Reche P, Ahmed AR (2011) Role of MHC Class II genes in the pathogenesis of pemphigoid. Autoimmun Rev 11:40–47
Zaraa I, Kerkeni N, Ishak F et al (2011) Spectrum of autoimmune blistering dermatoses in Tunisia: an 11-year study and a review of the literature. Int J Dermatol 50:939–944
Zillikens D, Wever S, Roth A et al (1995) Incidence of autoimmune subepidermal blistering dermatoses in a region in central Germany. Arch Dermatol 131:957–958
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alpsoy, E., Akman-Karakas, A. & Uzun, S. Geographic variations in epidemiology of two autoimmune bullous diseases: pemphigus and bullous pemphigoid. Arch Dermatol Res 307, 291–298 (2015). https://doi.org/10.1007/s00403-014-1531-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-014-1531-1