Abstract
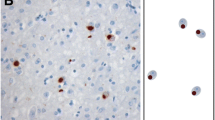
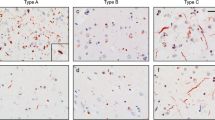
We have investigated the extent and pattern of immunostaining for the TAR DNA-binding protein, TDP-43, in 37 patients with frontotemporal lobar degeneration with ubiquitin (UBQ) pathology (FTLD-U). We confirm that TDP-43 protein is a component of the UBQ immunoreactive (UBQ-ir) neuronal cytoplasmic inclusions (NCI), neuronal intranuclear inclusions (NII) and neurites of the cerebral cortex and hippocampus in FTLD-U. We further show that the same three histological patterns, previously identified by us according to the form, number and distribution of the UBQ-ir NCI, NII and neurites are equivalently present in TDP-43 immunohistochemistry. TDP-43 immunoreactive (TDP-43-ir) NCI with rounded, spicular or skein-type appearance were seen in motor neurones of the trigeminal or facial cranial nerve nuclei in one patient with frontotemporal dementia (FTD) and in the spinal cord in three patients with FTD + motor neurone disease (MND). In patients with MND alone, TDP-43-ir NCI are common in anterior horn cells of the spinal cord, and occasionally seen in neurones of the hypoglossus nucleus. We show that TDP-43-ir NCI are also present within neurones in the superior and inferior olives in FTLD-U, and in some patients with MND. Although TDP-43 is normally seen as a nuclear protein, nuclear TDP-ir was not observed in neurones of the cerebral cortex, brainstem and spinal cord in FTLD-U or MND when NCI were present. We conclude that the UBQ-ir lesions of FTLD and MND are defined by the presence of TDP-43, and that these disorders can be subsumed into a single disease entity under the umbrella of TDP-43 proteinopathy.




Similar content being viewed by others
References
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Baker M, Mackenzie IRA, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M (2006) Mutations in Progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919
Bergmann M, Kuchelmeister K, Schmid KW, Kretzschmar HA, Schroder R (1996) Different variants of frontotemporal dementia: a neuropathological and immunohistochemical study. Acta Neuropathol 92:170–179
Boeve BF, Baker M, Dickson DW, Parisi JE, Giannini C, Jsephs KA, Hutton M, Pickering-Brown SM, Rademakers R, Tang-Wai D, Jack CR, Kantarci K, Shiung MM, Golde T, Smith GE, Geda YE, Knopman DS, Petersen RC (2006) Frontotemporal dementia and parkinsonism associated with the IVS1 + 1G→A mutation in progranulin: a clinicopathologic study. Brain 129:3103–3114
Brun A, Englund E, Gustafson L, Passant U, Mann DMA, Neary D, Snowden JS (1994) Clinical, neuropsychological and neuropathological criteria for fronto-temporal dementia. J Neurol Neurosurg Psychiatr 57:416–418
Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin J-J, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cujit I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442:920–924
Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, Chatterjee A, Hurtig HI, Karlawish JH, Rosen HJ, Van Deerlin V, Lee V M-Y, Miller BL, Trojanowski JQ, Grossman M (2006) Frontotemporal dementia: clinicopathological correlations. Ann Neurol 59:952–962
Froelich Fabre S, Axelman P, Almkvist A, Basun H, Lannfelt L (2003) Extended investigation of tau and mutation screening of other candidate genes on chromosome 17q21 in a Swedish FTDP-17 family. Am J Med Genet Part B (Neuropsychiatric Genet) 121B:112–118
Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, Kril JJ, Halliday GM (2004) Clinicopathological correlates in frontotemporal dementia. Ann Neurol 56:399–406
Josephs KA, Holton JL, Rossor MN, Godbolt AK, Osawa T, Strand K, Khan N, Al-Sarraj S, Revesz T (2004) Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol 30:369–373
Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, Parisi JE, Dickson DW (2006) Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 66:41–48
Katsuse O, Dickson DW (2005) Ubiquitin immunohistochemistry of frontotemporal lobar degeneration differentiates cases with and without motor neurone disease. Alzheimer Dis Assoc Disord 19(Suppl 1):S37–S43
Kovari E, Gold G, Giannakopoulos P, Bouras C (2004) Cortical ubiquitin-positive inclusions in frontotemporal dementia without motor neurone disease: a quantitative immunocytochemical study. Acta Neuropathol 108:207–212
Leigh PN, Anderton BH, Dodson A, Gallo J_M, Swash M, Power DM (1988) Ubiquitin deposits in anterior horn cells in motor neurone disease. Neurosci Lett 93:197–203
Lipton AM, White CL III, Bigio EH (2004) Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol 108:379–385
Lowe JS, Lennox G, Jefferson D, Morrell K, McQuire D, Gray T, Landon M, Doherty FJ, Mayer RJ (1988) A filamentous inclusion body within anterior horn neurones in motor neurone disease detected by immunocytochemical localisation of ubiquitin. Neurosci Lett 94:203–210
Mackenzie IRA, Feldman H (2003) The relationship between extramotor ubiquitin-immunoreactive neuronal inclusions and dementia in motor neurone disease. Acta Neuropathol 105:98–102
Mackenzie IRA, Feldman HH (2005) Ubiquitin immunohistochemistry suggests classic motor neuron disease, motor neuron disease with dementia and Frontotemporal dementia of the motor neuron disease type represent a clinicopathologic spectrum. J Neuropathol Exp Neurol 64:730–739
Mackenzie IRA, Baborie A, Pickering-Brown SM, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DMA (2006) Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration. Acta Neuropathol 112:539–549
Mackenzie IRA, Baker M, Pickering-Brown S, Hsiung G-Y R, Lindholm C, Dwosh E, Gass J, Cannon A, Rademakers R, Hutton M, Feldman H (2006) The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 129:3081–3090
Mackenzie IR, Baker M, West G, Woulfe J, Qadi N, Gass J, Cannon A, Adamson J, Feldman H, Lindholm C, Melquist S, Pettman R, Sadovnick AD, Dwosh E, Whiteheart SW, Hutton M, Pickering-Brown SM (2006) A family with tau-negative dementia and neuronal intranuclear inclusions linked to chromosome 17. Brain 129:853–867
Mackenzie IRA, Shi J, Shaw CL, Du Pleassis D, Neary D, Snowden D, Mann DMA (2006) dementia lacking distinctive histology (DLDH) revisited. Acta Neuropathol 112:551–559
Mann DMA (2005) The genetics and molecular pathology of frontotemporal lobar degeneration. In: Burns A, O’Brien J, Ames D (eds) Dementia, 3rd edn. Hodder Arnold, London, pp 689–701
McKhann GM, Albert MS, Grossman M, Miller B, Dickson DW, Trojanowski JQ (2001) Workgroup on Frontotemporal dementia and Pick’s disease; clinical and pathological diagnosis of frontotemporal dementia: report of the Workgroup on Frontotemporal dementia and Pick’s disease. Arch Neurol 59:1203–1204
Mott RT, Dickson DW, Trojanowski JQ, Zhukareva V, Lee VM, Forman M, Van Deerlin V, Ervin JF, Wang DS, Schmechel DE, Hulette CM (2005) Neuropathologic, biochemical, and molecular characterization of the frontotemporal dementias. J Neuropathol Exp Neurol 64:420–428
Mukherjee O, Pastor P, Cairns NJ, Chakraverty S, Kauwe JSK, Shears S, Behrens MJ, Budde J, Hinrichs AL, Norton J, Levich D, Taylor-Reinwald L, Gitcho M, Tu P-H, Grinberg LT, Liscic RM, Armendariz J, Morris JC, Goate AM (2006) HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Ann Neurol 60:314–322
Neary D, Snowden JS, Mann DMA (2005) Frontotemporal dementia. Lancet Neurol 4:771–779
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuk T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar H, Trojanowski JQ, Lee V M-Y (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Pickering-Brown SM, Baker M, Gass J, Boeve BF, Loy CT, Brooks WS, Mackenzie IR, Martins RN, Kwok JB, Halliday GM, Kril JJ, Schofield PR, Mann DM, Hutton M (2006) Mutations in progranulin explain atypical phenotypes with variants in MAPT. Brain 129:3124–3126
Rademakers R, Cruts M, Dermaut B, Sleegers K, Rosso SM, Van Den Broeck M, Backhovens H, van Swieten JC, van Duijn CM, van Broeckhoven C (2002) Tau-negative frontal lobe dementia at 17q21: significant finemapping of the candidate region to a 4.8-cm interval. Mol Psychiatr 7:1064–1074
Rosso SM, Kamphorst W, de Graaf B, Willemsen R, Ravid R, Niermeijer MF, Spillantini MG, Heutink P, van Swieten JC (2001) Familial frontotemporal dementia with ubiquitin positive inclusions is linked to chromosome 17q21–22. Brain 124:1948–1957
Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee V-M Y (2006) Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 169:1343–1352
Shi J, Shaw CL, Richardson AMT, Bailey K, Tian J, Varma AR, Neary D, Snowden JS, Mann DMA (2005) Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation. Acta Neuropathol 110:501–512
Shiarli AM, Jennings R, Shi J, Bailey K, Davidson Y, Tian J, Bigio E, Ghetti B, Arima K, Iseki E, Murayama S, Kretzchmar H, Lippa C, Halliday G, MacKenzie J, Khan N, Ravid R, Dickson D, Wszolek Z, Iwatsubo T, Pickering-Brown SM, Mann DMA (2006) Comparative tau pathology in Frontotemporal Lobar Degeneration and Alzheimer’s disease. Neuropathol Appl Neurobiol 32:374–387
Snowden JS, Neary D, Mann DMA (1996) Fronto-temporal lobar degeneration: fronto-temporal dementia, progressive aphasia, semantic dementia. Churchill Livingstone, Edinburgh pp 1–227
Snowden JS, Pickering-Brown SM, Mackenzie IR, Richardson AMT, Varma A, Neary D, Mann DMA (2006) Progranulin gene mutations associated with frontotemporal dementia and progressive aphasia. Brain 129:3091–3102
Taniguchi S, McDonagh AM, Pickering-Brown SM, Umeda Y, Iwatsubo T, Hasegawa M, Mann DMA (2004) The neuropathology of frontotemporal lobar degeneration with respect to the cytological and biochemical characteristics of tau protein. Neuropathol Appl Neurobiol 30:1–18
Woulfe J, Kertesz A, Munoz D (2001) Frontotemporal dementia with ubiquitinated cytoplasmic and intranuclear inclusions. Acta Neuropathol 102:94–102
Acknowledgment
We wish to acknowledge the technical assistance and expertise of Bev Dupuis, Lisa Laurence, Margaret Luk and Carmen Michelsen, the immunohistochemistry laboratory technologists at Vancouver General Hospital. Dr Pickering-Brown is the recipient of a Medical Research Council (MRC) New Investigator Award, and he and Professor Mann receive other funding from MRC, Alzheimers Research Trust and the Motor Neurone Disease Association. Funding support for Dr. Mackenzie was provided by the Canadian Institutes of Health Research (CIHR) grant no. 74580. The work of the Manchester Brain Bank is supported in part by the Alzheimers Research Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davidson, Y., Kelley, T., Mackenzie, I.R.A. et al. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol 113, 521–533 (2007). https://doi.org/10.1007/s00401-006-0189-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0189-y