Abstract
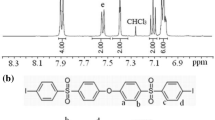
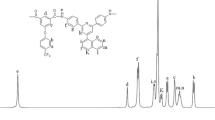
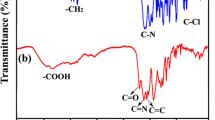
Aromatic polyamide nanoparticles with carbonyl chloride (COCl) and carboxyl (COOH) groups are prepared in a one-step precipitation polymerization process using a diamine and a diacid chloride, employing a combination of various solvents. The morphology, number of COCl groups introduced and molecular weight of the resulting particles are found to be greatly affected by the composition of the reaction solution. Pyridine acts primarily as a catalyst and also reduces the particle size and affects the incorporation of COCl groups. These groups originates from the diacid chloride and are present at the ends of polymeric chains. The solubility of the particles in the reaction solution is closely correlated with the time required for precipitation and significantly influences the particle formation mechanism. This in turn affects the molecular weight and the number of COCl groups.

Scheme of formation of aromatic polyamide nanoparticles with multiple functional groups












Similar content being viewed by others
References
Mohy Eldin MS, Shoroen CGPH, Janssen AEM, Mita DG, Tramper J (2000) Immobilization of penicillin G acylase onto chemically grafted nylon particles. J Mol Catal B Enzym 10:445–451
Lin C, Zhang Z, Zheng J, Liu M, Zhu XX (2004) Crosslinked polyacrolein microspheres with high loading of aldehyde groups for use as scavenger resins in organic synthesis. Macromol Rapid Commun 25:1719–1723
Vennes M, Zentel R (2004) Liquid-crystalline colloidal particles. Macromol Chem Phys 205:2303–2311
Holzapfel V, Musyanovych A, Landfester K, Lorenz MR, Mailander V (2005) Preparation of fluorescent carboxyl and amino functionalized polystyrene particles by miniemulsion polymerization as markers for cells. Macromol Chem Phys 206:2440–2449
Imbert-Laurenceau E, Berger M-C, Pavon-Djavid G, Jouan A, Migonney V (2005) Surface modification of polystyrene particles for specific antibody adsorption. Polymer 46:1277–1285
Nie C, Peng Z, Yang Y, Cheng C, Ma L, Zhao C (2006) Kevlar based nanofibrous particles as robust, effective and recyclable absorbents for water purification. J Hazard Mater 318:255–265
Zha T, Song L, Chen P, Nie W, Zhou Y (2015) Nonsolvent/solvent-induced phase separation to multi-porous sulfonated polystyrene/chitosan/silver particles and their application in adsorbing chromium ion(III) and reduction of methylene blue. Colloids Surf A Physicochem Eng Asp 481:423–430
Kang K, Kan CY, Du Y, Liu DS (2004) Control of particle size and carboxyl group distribution in soap-free emulsion copolymerization of methyl methacrylate-ethyl acrylate-acrylic acid. J Appl Polym Sci 92:433–438
Yoshioka Y (2011) Analysis and characterization of aromatic polyamide particles with trifluoromethyl and amino groups. Int J Polym Anal Charact 16:551–560
Kohri M, Nannichi Y, Kohma H, Abe D, Kojima T, Taniguchi T, Kishikawa K (2014) Size control of polydopamin nodules formed on polystyrene particles during dopamine polymerization with carboxylic acid-containing compounds for the fabrication of raspberry-like particles. Colloids Surf A Physicochem Eng Asp 449:114–120
Tsubokawa N, Oyanagi T (1993) Surface grafting of polymer onto aramid powder: reaction of chlorotriazinyl groups on the powder surface with functional polymers having terminal hydroxyl or amino groups. J Polym Sci A Polym Chem 31:1633–1637
Gelinas S, Finch JA, Vreugdenhil AJ (2000) Coupling of diethylenetriamine to carboxyl-terminated magnetic particles. Colloids Surf A Physicochem Eng Asp 164:257–266
Song J-S, Chagal L, Winnik MA (2006) Monodisperse micrometer-sized carboxyl-functionalized polystyrene particles obtained by two-stage dispersion polymerization. Macromolecules 39:5729–5737
Lappan U, GeiBler U (2008) PTFE micropowder functionalized with carboxylic acid groups. Macromol Mater Eng 293:538–542
Yoshsioka Y, Asao K (2008) Preparation of nano-sized polybenzimidazole and carbon particles via poly(amino-amide) particles. Colloid Polym Sci 286:1157–1164
Minami F, Yamamoto S, Miyasaka Y, Moriya O (2011) Synthesis of thermo- and pH-responsive polysilsesquioxane with carboxylic acid group. Polymer 52:4744–4752
Meder F, Daberkow T, Treccani L, Whihelm M, Schowalter M, Rosenauer A, Madler L, Rezwan K (2012) Protein adsorption on colloidal alumina particles functionalized with amino, carboxyl, sulfonate and phosphate groups. Acta Biomater 8:1221–1229
Majewski P, Albrecht T, Weber S (2011) COOH-functionalisation of silica particles. Appl Surf Sci 257:9282–9286
Bayramoglu G, Arica MY (2016) MCM-41 silica particles grafted with polyacrylonitrile: modification in to amidoxime and carboxyl groups for enhanced uranium removal from aqueous medium. Microporous Mesoporous Mater 226:117–124
Yoshioka Y, Asao K, Yamamoto K, Tachi H (2007) Preparation of micron-sized and aromatic polyamide particles using ultrasonic irradiation. Colloid Polym Sci 285:535–541
Kim PK, Chang C, Hsu SL (1986) Normal vibration analysis of a rigid rod polymer: poly(p-phenylene terephthalamide). Polymer 27:34–46
Snyder RW, Thomson B, Bartges B, Czerniawski D, Painter PC (1989) FTIR studies of polyimides: thermal curing. Macromolecules 22:4166–4172
Stuart BH (1995) A Fourier transform Raman study of water sorption by Kevlar-49. Polym Bull 35:727–733
Yoshioka Y, Tashiro K, Ramesh C (2003) Structural change in the Brill transition of nylon m/n (2) conformational disordering as viewed from the temperature-dependent infrared spectral measurements. Polymer 44:6407–6417
Yoshioka Y, Asao K, Yamamoto K, Tachi H (2007) Preparation and characterization of nanoscale aromatic polyamide particles. Polymer 48:2214–2220
Lin-Vien D, Colthup NB, Fateley WG, Grasselli JG (1991) The handbook of infrared and Raman characteristic frequencies of organic molecules. Academic, California, p 125
Socrates G (1991) Infrared and Raman characteristic group frequencies. John Wiley & Sons, Chichester, p 149
Acknowledgements
The author thanks Dr. Tomoko Nakahashi of Osaka Research Institute of Industrial Science and Technology for the cooperation in the GPC measurements.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they has no conflict of interest.
Rights and permissions
About this article
Cite this article
Yoshioka, Y. Preparation of aromatic polyamide nanoparticles with multiple functional groups in mixed solvent solutions via a one-step precipitation polymerization. Colloid Polym Sci 296, 1657–1666 (2018). https://doi.org/10.1007/s00396-018-4388-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4388-6