Abstract
Simple analytic expressions are derived for the electrostatic interaction energy between two charged colloidal particles in an electrolyte solution. The obtained expressions are based on an approximate form of the modified Poisson-Boltzmann equation taking into account the ion size effects through Carnahan-Starling activity coefficients of electrolyte ions. We derive the electrostatic interaction energy between two parallel plates on the basis of the linear superposition approximation. We further employ Derjaguin’s approximation to derive the corresponding expressions for the electrostatic interaction energy between two spheres, two parallel cylinders, or two crossed cylinders.


Similar content being viewed by others
References
Derjaguin BV, Landau L (1941) Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim USSR 14:633–662
Verwey EJW, Overbeek JTG (1948) Theory of the stability of lyophobic colloids. Elsevier/Academic Press, Amsterdam
Dukhin SS (1993) Non-equilibrium electric surface phenomena. Adv Colloid Interf Sci 44:1–134
Ohshima H, Furusawa K (eds) (1998) Electrical phenomena at interfaces, fundamentals, measurements, and applications, 2nd edition, revised and expanded. Dekker, New York
Delgado AV (ed) (2000) Electrokinetics and electrophoresis. Dekker, New York
Lyklema J (2005) Fundamentals of interface and colloid science, volume IV, chapter 3. Elsevier/Academic Press, Amsterdam
Ohshima H (2006) Theory of colloid and interfacial electric phenomena. Elsevier/Academic Press, Amsterdam
Ohshima H (2010) Biophysical chemistry of biointerfaces. Wiley, Hoboken
Ohshima H (ed) (2012) Electrical phenomena at interfaces and biointerfaces: fundamentals and applications in nano-, bio-, and environmental sciences. Wiley, Hoboken
Sparnaay MJ (1972) Ion-size corrections of the Poisson-Boltzmann equation. J Electroanal Chem 37:65–70
Adamczyk Z, Warszyński P (1996) Role of electrostatic interactions in particle adsorption. Adv in Colloid Interface Sci 63:41–149
Biesheuvel PW, van Soestbergen M (2007) Counterion volume effects in mixed electrical double layers. J Colloid Interface Sci 316:490–499
Lopez-Garcia JJ, Horno J, Grosse C (2011) Poisson-Boltzmann description of the electrical double layer including ion size effects. Langmuir 27:13970–13974
Lopez-Garcia JJ, Horno J, Grosse C (2012) Equilibrium properties of charged spherical colloidal particles suspended in aqueous electrolytes: finite ion size and effective ion permittivity effects. J Colloid Interface Sci 380:213–221
Giera B, Henson N, Kober EM, Shell MS, Squires TM (2015) Electric double-layer structure in primitive model electrolytes: comparing molecular dynamics with local-density approximations. Langmuir 31:3553–3562
Lopez-Garcia JJ, Horno J, Grosse C (2016) Ion size effects on the dielectric and electrokinetic properties in aqueous colloidal suspensions. Curr Opinion in Colloid Interface 24:23–31
Bikerman JJ (1942) Structure and capacity of electrical double layer. Philos Mag 33:384–397
Hill TL (1962) Statistical thermodynamics. Addison-Westley, Reading, MA
Carnahan NF, Starling KE (1969) Equation of state for nonattracting rigid spheres. J Chem Phys 51:635–636
Ohshima H (2016) An approximate analytic solution to the modified Poisson-Boltzmann equation. Effects of ionic size. Colloid Polym Sci 294:2121–2125
Ohshima H, Healy TW, White LR (1984) Accurate analytic expressions for the surface charge density/surface potential relationship and double-layer potential. J Colloid Interface Sci 90:17–26
Wilemski G (1982) Weak repulsive interactions between dissimilar electrical double layers. J Colloid Interface Sci 88:111–116
Ohshima H (1995) Effective surface potential and double-layer interaction of colloidal particles. J Colloid Interface Sci 174:45–52
Derjaguin BV (1934) Untersuchungen über die Reibung und Adhäsion, IV. Kolloid Z 69:155–164
Sparnaay MJ (1959) The interaction between two cylinder shaped colloidal particles. Recueil 78:680–709
Ohshima H, Hyono A (2009) Electrostatic interaction between two cylindrical soft particles. J Colloid Interface Sci 333:202–208
Ohshima H (2014) Approximate analytic expression for the stability ratio of colloidal dispersions. Colloid Polym Sci 292:2269–2274
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Source of funding
The author declares no sources of funding.
Appendix
Appendix
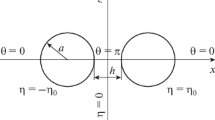
Analytic expressions for the interaction between two parallel similar plates at separation h can be derived on the basis of the modified Poisson-Boltzmann equation (19). The interaction force P pl(h) per unit area between the two plates is given by
with
where y m is the scaled potential at the midpoint x = h/2 between the two plates. Eq. (52) can be derived from Eq. (35) by choosing h/2 as x′. Note that Eq. (52) can be applied for both of the constant surface potential and surface charge density models. For the constant surface potential model, y m is related to the scaled surface potential y o = zeψ o /kT, viz.,
which is derived by applying Eq. (20) for the system of two parallel plates. For the constant surface charge density model, on the other hand, we have
Note that the scaled surface potential y (0), which is a function of the plate separation h, is related to the scaled unperturbed surface potential y o = zeψ o /kT in the absence of interaction (h = ∞) by
which can be derived by integrating Eq. (19) once and applying the boundary condition: dψ/dx| x=0 + = − σ/ε r ε o and dψ/dx| x = h/2 = 0 (from symmetry of the system). The surface charge density σ of the plates is related to the scaled unperturbed surface potential y o = zeψ o /kT (in the absence of interaction) by
By combining Eqs. (56) and (57), we obtain
Equations (55) and (58) form coupled equations for y m for the given values of y o and κh.
Once the value of y m is obtained from Eq. (54) for the constant potential model (in which y(0) is always equal to the unperturbed surface potential y o) or the coupled Eqs. (55) and (58) for the constant surface charge density model, one can calculate the interaction force P pl(h) via Eq. (52).
Rights and permissions
About this article
Cite this article
Ohshima, H. Approximate analytic expressions for the electrostatic interaction energy between two colloidal particles based on the modified Poisson-Boltzmann equation. Colloid Polym Sci 295, 289–296 (2017). https://doi.org/10.1007/s00396-016-4005-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-4005-5