Abstract
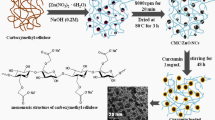
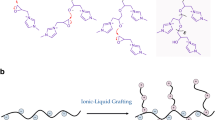
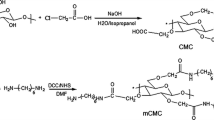
In this paper, hexamethylene diamine (HMDA)-grafted carboxymethyl cellulose (CMC/HMDA) was synthesized through the direct graft reaction of HMDA with carboxymethyl cellulose (CMC). Then the folic acid (FA) was covalently linked to the CMC/HMDA to produce FA (folate)-conjugated CMC/HMDA (CMC/HMDA-FA). The final copolymer was confirmed by Fourier transform infrared spectroscopy (FT-IR), Ultraviolet–visible spectroscopy (UV–vis), proton nuclear magnetic resonance (1H NMR) spectroscopy, thermal gravimetric analysis (TGA), and scanning electron microscopy (SEM). Doxorubicin (DOX) as a model anticancer drug was used and in vitro DOX release from the CMC/HMDA-FA in two different media (bicarbonate buffer solutions: pH = 5 and pH = 7.4) was studied. The results were indicated that the release speed of DOX could be well controlled and the release period could be reached up to 12 h. Release profiles of the DOX from the CMC/HMDA-FA and its loading capacity were determined by UV–vis absorption measurement at λ max 483 nm. These results suggested that the CMC/HMDA-FA conjugate would be beneficial for an anti-tumor drug carrier for pharmaceutical applications.







Similar content being viewed by others
References
Nichifor M, Mocanu G (2006) Polysaccharides for drug delivery and pharmaceutical applications. In: Marchessault RH, Ravenelle F, Zhu XX (eds) Polysaccharides for drug delivery and pharmaceutical applications. Oxford University, pp 289–pp 303
Chen S, Zhang XZ, Cheng SX, Zhuo RX, Gu ZW (2008) Functionalized amphiphilic hyperbranched polymers for targeted drug delivery. Biomacromolecules 9:2578–2585. doi:10.1021/bm800371n
Park JH, Lee S, Kim JH, Park K, Kim K, Kwon IC (2008) Polymeric nanomedicine for cancer therapy. Prog Polym Sci 33:113–137. doi:10.1016/j.progpolymsci.2007.09.003
Van Vlerken LE, Vyas TK, Amiji MM (2007) Poly (ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm Res 24:1405–1414. doi:10.1007/s11095-007-9284-6
Zhang C, Zhao LQ, Dong YF, Zhang XY, Lin J, Chen Z (2010) Folate-mediated poly (3-hydroxybutyrate-co-3-hydroxyoctanoate) nanoparticles for targeting drug delivery. Eur J Pharm Biopharm 76:10–16. doi:10.1016/j.ejpb.2010.05.005
Schneider R, Schmitt F, Frochot C, Fort Y, Lourette N, Guillemin F, Müller JF, Barberi-Heyob M (2005) Design, synthesis, and biological evaluation of folic acid targeted tetraphenylporphyrin as novel photosensitizers for selective photodynamic therapy. Bioorg Med Chem 13:2799–2808. doi:10.1016/j.bmc.2005.02.025
Yang XQ, Chen YH, Yuan RX, Chen GH, Blanco E, Gao JM, Shuai XT (2008) Folate-encoded and Fe3O4-loaded polymeric micelles for dual targeting of cancer cells. Polymer 49:3477–3485. doi:10.1016/j.polymer.2008.06.005
Husseini GA, Pitt WG (2008) Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv Drug Deliv Rev 60:1137–1152. doi:10.1016/j.addr.2008.03.008
Li R, Feng F, Wang Y, Yang X, Yang X, Yang VC (2014) Folic acid-conjugated pH/temperature/redox multi-stimuli responsive polymer microspheres for delivery of anti-cancer drug. Colloid Interface Sci 429:34–44. doi:10.1016/j.jcis.2014.05.008
Wei K, Peng X, Zou F (2014) Folate-decorated PEG–PLGA nanoparticles with silica shells for capecitabine controlled and targeted delivery. Int J Pharm 464:225–233. doi:10.1016/j.ijpharm.2013.12.047
Yan CY, Gu JW, Hou DP, Jing HY, Wang J, Guo YZ, Katsumi H, Sakane T, Yamamotoca A (2015) Synthesis of Tat tagged and folate modified N-succinyl-chitosan self-assembly nanoparticles as a novel gene vector. Int J Biol Macromolec 72:751–756. doi:10.1016/j.ijbiomac.2014.09.031
Kumari A, Yadav SK, Yadav SC (2010) Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf 75:1–18. doi:10.1016/0927-7757(93)80410-G
Memvanga PB, Préat V (2012) Formulation design and in vivo antimalarial evaluation of lipid-based drug delivery systems for oral delivery of beta-arteether. Eur J Pharm Biopharm 82:112–119. doi:10.1016/j.ejpb.2012.05.004
Movgharnezhad N, Najafi Moghadam P (2014) Synthesis of methoxy poly (ethylene glycol)/starch grafted copolymers and investigation of their drug release behavior. Starch – Stärke 66:1–7. doi:10.1002/star.201400044
Tan ML, Choong PFM, Dass CR (2009) Review: doxorubicin delivery systems based on chitosan for cancer therapy. J Pharm Pharmacol 61:131–142. doi:10.1211/jpp/61.02.0001
Bochek AM (2003) Effect of hydrogen bonding on cellulose solubility in aqueous and nonaqueous solvents. Russ J Appl Chem 76:1711–1719. doi:10.1023/B:RJAC.0000018669.88546.56
Hinterstoisser B, Salmen L (2000) Application of dynamic 2D FTIR to cellulose. Vib Spectrosc 22:111–118. doi:10.1016/S0924-2031(99)00063-6
Liua H, Ilevbare GA, Cherniawski BP, Ritchie ET, Taylor LS, Edgar KJ (2014) One-step fabrication of biocompatible carboxymethyl cellulose polymeric particles for drug delivery systems. Carbohydr Polym 100:116–125. doi:10.1016/j.carbpol.2011.05.001
Meng T, Gao X, Zhang J, Yuan JY, Zhang YZ, He JS (2009) Graft copolymers prepared by atom transfer radical polymerization (ATRP) from cellulose. Polymer 50:447–454. doi:10.1016/j.polymer.2008.11.011
Wang D, Tan J, Kang H, Ma L, Jin X, Liu R, Huang Y (2011) Synthesis, self-assembly and drug release behaviors of pH-responsive copolymers ethyl cellulose-graft-PDEAEMA through ATRP. Carbohydr Polym 84:195–202. doi:10.1016/j.carbpol.2010.11.023
Buzzi V, Brudner M, Wagner TM, Bazzo GC, Pezzin APT, Silva DAK (2013) Caboxymetylcellulose/gelatin blends loaded with piroxicam: preparation, characterization and evaluation of in vitro release profile. J Encap Ads Sci 3:99–107. doi:10.4236/jeas.2013.34012
Davidson DW, Verma MS, Gu FX (2013) Controlled root targeted delivery of fertilizer using an ionically crosslinked carboxymethyl cellulose hydrogel matrix. Springer Plus 2:318–326. doi:10.1186/2193-1801-2-318
Diftis N, Kiosseoglou V (2003) Improvement of emulsifying properties of soybean protein isolate by conjugation with carboxymethyl cellulose. Food Chem 81:1–6. doi:10.1016/S0308-8146(02)00236-4
Jabbour L, Bongiovanni R, Chaussy D, Gerbaldi C, Beneventi D (2013) Cellulose-based Li-ion batteries: a review. Cellulose 20:1523–1545. doi:10.1007/s10570-013-9973-8
Tiitu M, Laine J, Serimaa R, Ikkala O (2006) Ionically self-assembled carboxymethylcellulose-surfactant complexes for antistatic paper coatings. J Colloid Interface Sci 301:92–97. doi:10.1016/j.jcis.2006.04.072
Liu J, He F, Gunn TM, Zhao D, Roberts CB (2009) Precise seed-mediated growth and size-controlled synthesis of palladium nanoparticles using a green chemistry approach. Langmuir 25:7116–7128. doi:10.1021/la900228d
Yoshimura T, Matsuo K, Fujioka R (2006) Novel biodegradable superabsorbent hydrogels derived from cotton cellulose and succinic anhydride: synthesis and characterization. J Appl Polym Sci 99:3251–3256. doi:10.1002/app.22794
Yang X, Liu Q, Chen X, Yu F, Zhu Z (2008) Investigation of PVA/ws-chitosan hydrogels prepared by combined gamma-irradiation and freeze-thawing. Carbohyd Polym 73:401–408
Nagasawa N, Yagi T, Kume T, Yoshii F (2004) Radiation crosslinking of carboxymethyl starch. Carbohydr Polym 58:109–113
Gupta P, Vermani K (2002) Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today 7:569–579
Cheng Y, Lu L, Zhang W, Shi J, Cao Y (2012) Reinforced low density alginate-based aerogels: preparation, hydrophobic modification and characterization. Carbohydr Polym 88:1093–1099. doi:10.1016/j.carbpol.2012.01.075
Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S (2009) Folate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn® H40, poly (llactide) and poly (ethylene glycol) for tumor-targeted drug. Biomaterials 30:3009–3019. doi:10.1016/j.biomaterials.2009.02.011
Qiu LY, Wang RJ, Zheng C, Jin Y, Jin LQ (2010) ß-cyclodextrin-centered star-shapedamphiphilic polymers for doxorubicin delivery. Nanomedicine 5:193–208. doi:10.2217/nnm.09.108
Acknowledgments
The authors wish to acknowledge financial supports from the University of Urmia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Movagharnezhad, N., Moghadam, P.N. Folate-decorated carboxymethyl cellulose for controlled doxorubicin delivery. Colloid Polym Sci 294, 199–206 (2016). https://doi.org/10.1007/s00396-015-3768-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3768-4