Abstract
A nonaqueous ionic liquid (IL) microemulsion containing ethylammonium nitrate (EAN), the surfactant TX-100, and cyclohexane was prepared at room temperature, and phase behavior of this ternary system was investigated at 25 ± 0.1 °C. Electric conductivity measurements were performed to identify the microregions of the nonaqueous IL microemulsion. Based on percolation theory, an EAN-in-cyclohexane (IL/O), a bicontinuous, and a cyclohexane-in-EAN (O/IL) microregion were successfully identified using cyclohexane as the titration phase. Dynamic light scattering measurements were used to study the formation of the IL pools. UV–vis spectroscopy using methyl orange as probe material was employed to investigate the micropolarity of the system. The results indicate that the micropolarity of the IL/O microemulsion increases with the EAN content after the IL pools are formed. With this work, we expect to improve the basic understanding of nonaqueous microemulsions.
Similar content being viewed by others
Introduction
Microemulsions are thermodynamically stable, isotropic systems, usually containing three components: water, oil, and surfactant. In addition, a typical conventional microemulsion always contains alcohol as a cosurfactant. The surfactants adsorb at the interface of oil and water, separating two liquid phases by forming an elastic film at the interface [1]. The current prevalence in studying microemulsions can be attributed to the occurrence of special microstructures, including oil-in-water (O/W) or water-in-oil (W/O) microdroplets at the nanometer scale, and bicontinuous microstructures [2, 3]. The unique microstructure of microemulsions provides an ideal environment for reactions and nanoparticle preparation [4, 5]. Microemulsions have also been extensively applied to other fields, e.g., for controlling chemical reactions and drug delivery [6–8]. However, for some water-sensitive reactions, such as esterification and polymerization, it is necessary to substitute the water phase with other solvents in order to create a nonaqueous microenvironment [9].
Due to their excellent properties, ionic liquids (ILs) are a promising candidate material for replacing water. In contrast to common inorganic salts, such as sodium chloride (NaCl), ILs are organic salts with a melting temperature below 100 °C. The low melting temperature is attributed to the presence of sterically mismatched ions that hinder crystal formation [10–15]. The first study on ILs can be dated back to 1914 and reported on the synthesis of ethylammonium nitrate (EAN) [16]. Recently, studies on ILs have received much attention [17–21]. The favorable properties of these ILs, such as their nonvolatility, a high stability, a suitable polarity, and their high conductivity, make them perfect alternatives to conventional solvents. Due to these excellent properties, ILs are regarded as “environmentally friendly solvents” and have been used as suitable solvents for the separation of liquids and for electrochemical applications [22, 23]. Recent developments involve their application as a component in microemulsions, especially nonaqueous microemulsions [24–28].
In the past few years, nonaqueous microemulsions composed of ILs as well as their phase behavior have been extensively studied. EAN is a good substitute for water. Evans et al. first reported on the micelles formed by some common surfactants in EAN in the 1980s, and they later observed the formation of lyotropic liquid crystals in EAN [29–32]. The phase behavior of various nonionic surfactants in EAN was studied by Atkin et al., who also investigated microemulsions containing EAN as a polar phase [33, 34]. In addition to nonaqueous micelles and liquid crystals, the formation of nonaqueous microemulsions with the aid of ILs has also been widely reported. For instance, Gao et al. studied the structure of nonaqueous microemulsion containing 1-butyl-3-methylimidazolium tetrafluoroborate (BmimBF4), TX-100, and p-xylene [35]. Xu et al. investigated nonaqueous IL microemulsions composed of BmimBF4, toluene and ethanol in the absence of surfactant [36]. Mandal et al. studied the excitation wavelength-dependent fluorescence resonance energy transfer in a novel IL-in-oil microemulsion containing the biologically acceptable component isopropyl myristate [37].
The study of nonaqueous IL microemulsions may expand the application range of traditional microemulsions, while also promoting fundamental research. Furthermore, some potential applications, such as the control of chemical reactions, the synthesis of novel materials, and extractions and fractionations utilizing the nano-size IL environments, might arise due to the unique features of ILs and IL-based microemulsions.
Although nonaqueous microemulsions show some application potential, the potential toxicity of ILs as a limiting factor has been rarely investigated [38–40]. Bernot et al. studied the effect of several ILs on the survival and behavior (movement and feeding rates) of the freshwater pulmonate snail, Physa acuta. The snail was exposed to median lethal concentrations (LC50) of nine ILs with imidazolium- and pyridinium-based cations and Br− and PF6 − as anions in the range from 1 to 325 mg/L. Only two ILs were found to be highly toxic, while the other ILs were harmless [38]. As another example, Dai et al. studied the biodegradation of 1-octyl-3-methyl imidazolium hexafluorophosphate ([Omim][PF6]). At first, the biodegradation rate of [Omim][PF6] is lower than 20 %. However, after introducing the ester group to [Omim][PF6], the biodegradation rate of IL is increased to 60 % [39]. Carlo et al. have studied the toxicity of 15 different ILs and reported that most ILs (13) can be classified as nontoxic and the two toxic ILs were both quaternary ammonium salts [40]. The toxicity of ILs generally depends on their structure. When transferring an IL to practical application, the toxicity of the IL must first be evaluated to prevent any harm to the environment.
In this study, we investigated the phase behavior of the EAN/TX-100/cyclohexane ternary system. The microregions were identified by electric conductivity measurements, and dynamic light scattering (DLS) was used to further study the formation of the microemulsion. UV–vis spectroscopy was performed to investigate the micropolarity of the system with methyl orange (MO) used as the probe material.
Material and methods
Materials
EAN was prepared via the reaction of equimolar amounts of ethylamine and nitric acid, as described by Evans et al. [30]. TX-100, cyclohexane, and MO were obtained from Aladdin, China. TX-100 was dried in a vacuum at 80 °C for 4 h to remove any excess water prior to the experiments. To avoid absorption of air moisture, IL was freshly produced and further dehydrated before use by applying the same vacuum dehydration process.
Apparatus and procedures
The phase diagram of the system was firstly determined by direct visual observation at 25 ± 0.1 °C. For a typical experimental sample, EAN and cyclohexane were mixed in a glass test tube at different weight ratios, and then, TX-100 was added dropwise to the mixture until the cloudy and milky liquid became transparent, indicating the formation of an isotropic phase. The corresponding composition of the solution was recorded as the phase boundary.
Electric conductivity measurements were performed using a low-frequency (1 kHz) conductivity meter (Model DDS-307, Shanghai Technology & Instrument Co., Ltd., China) with a cell constant of 0.965 cm−1 and an accuracy of ±1 %. In the experiment, a thermostable glass test tube with a diameter of 20 mm and a length of 100 mm was used as the dip cell. The conductivity of the test tube can be ignored compared to the conductivity of the samples. The fixed amounts of EAN and TX-100 were mixed in the test tube at 25 ± 0.1 °C. Then, cyclohexane was added dropwise. After shaking the mixture and waiting until a uniform distribution was observed, the electric conductivity was measured after each addition of cyclohexane and recorded. Cyclohexane was successively added to the mixture until the transparent liquid solution became turbid.
The size of the microemulsion droplets was determined utilizing the DLS technique (Zetasizer Nano ZS, Malvern Instruments, UK) with a laser light wavelength of 633 nm and a scattering angle of 90°. The sample was prepared in a fume hood prior to the test to avoid possible pollution and filtered through 0.2-mm Millipore nylon filters to remove residual dust. The sample pool was ultrasonically cleaned with ultra-pure water after being dipped for 10 min with 1 % TX-100. Then, the sample pool was dried with nitrogen before the sample was transferred to the square sample pool. Each sample was measured three times to obtain precise data. About 100 data sets were obtained from each measurement. All measurements were performed at 25 ± 0.1 °C.
The UV–vis spectra were recorded using an UV-2802 spectrophotometer (UNICO Shanghai Instrument Co., Ltd. China) operated at a temperature of 25 ± 0.1 °C. The path length of the quartz cell used in this experiment was 1 cm, and the cell was rinsed prior to each scan. The samples were prepared in advance and then added to the quartz cell.
Results and discussion
Phase behavior of microemulsion
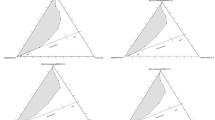
Determining the phase behavior is the most critical part in the study of microemulsions. The phase diagram of the EAN/TX-100/cyclohexane ternary system obtained at room temperature is shown in Fig. 1. A single isotropic region extending from IL corner to TX-100 corner can be observed. The upper shadow zone in Fig. 1 is the multiphase area, in which the microemulsion is in balance with the excess cyclohexane or EAN. The remaining area in the diagram is the single isotropic phase region. As seen in Fig. 1, a continuous stable single-phase microemulsion region was observed over the entire TX-100 or EAN content interval from 0 to 100 wt%. In conventional aqueous microemulsion systems, W/O microemulsion droplets are formed at lower water contents. With increasing water content, a microstructural transition can occur in the microemulsion if the system remains isotropic. We assume that a similar phase transition occurs in nonaqueous microemulsions. Therefore, it is interesting to investigate the microstructures of the microemulsion and their transition behavior.
Microregions of the microemulsion
In general, electric conductivity measurements can be employed to determine the microregions of microemulsions and phase transitions based on percolation theory. For conventional typical aqueous microemulsions, Clausse et al. previously reported that with increasing water content, the electrical conductivity κ of microemulsion changes in four successive stages: (1) an original nonlinear increase in κ, (2) the following linear increase, (3) the second nonlinear increase, and (4) a final decrease in κ [41]. The different stages of electrical conductivity are ascribed to the different microemulsion structures and phase transitions with the addition of water.
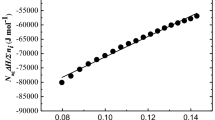
In this work, EAN was chosen as a substitute for water and cyclohexane as the oil phase. Compared with traditional aqueous microemulsion system, EAN obviously has excellent electrical conductivity. In the titrimetric analysis experiments of electrical conductivity, cyclohexane was used as the titration phase to determine the microstructure of microemulsion. Figure 2 shows the electrical conductivity versus added cyclohexane content at different TX-100/EAN weight ratios. The TX-100/EAN weight ratios are selected to be 5:5, 7:3, and 8:2 to 9:1 and marked by the four dotted lines in Fig. 1, which run from the horizontal axis to the cyclohexane corner in the single-phase region of phase diagram.
Figure 2a, b shows the electrical conductivity at the TX-100/EAN weight ratios of 5:5 and 7:3, respectively. From these two panels, the electrical conductivity κ always increases with the addition of cyclohexane, indicating the formation of O/IL microemulsion. This is due to the increase of movable ions in the microemulsion system. At the initial stage, a relatively small amount of dielectric nonpolar cyclohexane is added to the mixture. The cyclohexane is dissolved into the inner phase and the system is isotropic, resulting in the formation of O/IL microdroplets. The microdroplets disperse in EAN phase with TX-100 adsorbed at the interface of cyclohexane and EAN. With the further addition of cyclohexane, the viscosity of microemulsion decreases, thereby lowering the limitation of EAN movement. More ions become movable due to the formation of additional microdroplets, which results in the linear increase of the conductivity. When TX-100/EAN weight ratio is 8:2, the initial stage is similar to Fig. 2a, b. With further addition of cyclohexane to the mixture, as shown in Fig. 2c, the gradient of electric conductivity starts to decrease and the conductivity gradually reaches the maximum. This may be due to the reduction of the O/IL microdroplet formation rate. This microemulsion region is determined as the bicontinuous phase. At this stage, the interaction between the O/IL microdroplets is enhanced and a structural transition occurs in the system. Both EAN and cyclohexane are continuous phases. When the TX-100/EAN weight ratio is 9:1 as shown in Fig. 2d, with the increase of cyclohexane content, the initial tendency is similar to that of Fig. 2c. With further addition of cyclohexane, the electrical conductivity begins to decrease, indicating the formation of IL/O microdroplets. When cyclohexane is added in excess, the balance between EAN and cyclohexane is disturbed. Some IL/O microdroplets begin to form in the system, decreasing the number of movable ions. When more cyclohexane is added, the conductivity decreases as a result of the formation of additional IL/O microdroplets. The conductivity curves in Fig. 2 clearly determine three different microregions of microemulsion: O/IL, bicontinuous phase, and IL/O microregions.
Dynamic light scattering
DLS is a powerful tool to study the self-assembled aggregates, especially the microemulsion. According to the previous reports [1, 28], there exists a swelling law for the formation of microemulsion. When adding polar IL phase, the linear increase of microemulsion droplets radius with IL content is a powerful evidence for the solubilization of IL into the oil phase and the formation of IL-in-oil (IL/O) microemulsion (“IL pools”). This regular swelling behavior is due to the solubilization of IL discrete spherical droplets in the oil phase stabilized by the surfactant. If the radius of microemulsion droplets is nonlinear to the added amount of IL phase, this may be due to the interactions and polydispersity between IL droplets.
In this work, a fixed TX-100/cyclohexane molar ratio of 1.17:1 was chosen to study the EAN-in-cyclohexane microemulsion. Figure 3 shows the variation of the radius of microemulsion droplets with increasing EAN content. When the EAN content increases from 18.53 to 42.71 % (Fig. 3a–c), the radius of microemulsion droplets increases from 4.19 to 10.31 nm. With the increase of EAN content, the radius of IL pools increases, indicating the swelling behavior of IL pools. Figure 3d shows the radius of microemulsion droplets versus EAN content. There is a linear increase tendency of radius with the EAN content, which accords well with the swelling law of microemulsion droplets and in good agreement with previous studies reporting on the investigation of other IL microemulsions [27–29, 42]. This indicates the formation of discrete spherical IL pools in oil phase. For better understanding, Fig. 3e is proposed to show this swelling process of IL pools. With the addition of inner IL phase, the EAN-in-cyclohexane microemulsion phase is formed and the radius of droplets increases with the added amount of EAN, indicating the spherical structure droplets.
UV–vis spectroscopy
In order to further study the microstructure of the microemulsions, UV–vis spectroscopy was performed to investigate the microenvironment of EAN in the microemulsions. MO was chosen as the probe material to study the micropolarity of the polar domains in the microemulsion. The shift in the absorbance maximum of MO provides a sensitive measurement of the local environment. As a red-shift indicator, the maximum of the MO absorbance shifts to larger wavelengths when the polarity of the medium increases [43].
The obtained UV–vis spectra are shown in Fig. 4. For these experiments, the TX-100 content was fixed at 65 wt%. When the EAN/TX-100 molar ratio is increased from 0.5, 1.0, 1.8 to 2 (Fig. 4a–d), the maximum absorbance peak shifts from 415 to 420, 423 and 425 nm, respectively. This means that the micropolarity of the microemulsion droplets increases with the increase of EAN content, indicating the formation of IL pools in the microemulsion. Through the measurement of the UV–vis spectra, the IL/O microemulsion was further studied, and the results are in good agreement with the phase diagram.
Results of the UV–vis spectroscopy measurements for a fixed TX-100 content of 65 wt%. The EAN/TX-100 mole ratio of the samples a, b, c, and d was selected to 0.5, 1.0, 1.8 to 2, respectively, and the maximum absorbance peak for the samples a, b, c, and d was observed at 415, 420, 423, and 425 nm, respectively
Conclusion
In summary, the ternary system consisting of EAN, TX-100, and cyclohexane was studied and its phase behavior was investigated. The EAN-in-cyclohexane (IL/O), bicontinuous, and cyclohexane-in-EAN (O/IL) microregions of nonaqueous microemulsions were identified by electric conductivity measurements based on percolation theory. The obtained DLS results were in accordance with the swelling law of microemulsions and further confirmed the formation of microemulsion. In addition, UV–vis spectroscopy was performed using MO as probe material, revealing that the polarity of IL pools increases with the EAN content. We expect that this work can improve our knowledge of nonaqueous microemulsions and provide a new nonaqueous microemulsion system for novel potential applications in different fields, e.g., for the preparation of nanomaterials and chemical reactions.
References
Gao YA, Han SB, Han BX, Li GZ, Shen D, Li ZH, Du JM, Hou WG, Zhang GY (2005) TX-100/water/1-butyl-3-methylimidazolium hexafluorophosphate microemulsions. Langmuir 21:5681–5684
Mackay RA, Myers SA, Bodalbhai L (1990) Microemulsion structure and its effect on electrochemical reactions. Anal Chem 62:1084–1090
Gennes PG, Taupin C (1982) Microemulsions and the flexibility of oil/water interfaces. J Phys Chem 86:2294–2304
Marciano V, Minore A, Liveri VT (2000) A simple method to prepare solid nanoparticles of water-soluble salts using water-in-oil microemulsions. Colloid Polym Sci 278:250–252
Koetz J, Baier J, Kosmella S (2007) Formation of zinc sulfide and hydroxylapatite nanoparticles in polyelectrolyte-modified microemulsions. Colloid Polym Sci 285:1719–1726
Oh SG, Kizling J, Holmberg K (1995) Microemulsions as reaction media for the synthesis of sodium decyl sulfonate 1: role of microemulsion composition. Colloid Surf A 97:169–179
Spiro M, Jesus DM (2000) Nanoparticle catalysis in microemulsions: oxidation of N, N-dimethyl-p-phenylenediamine by cobalt(III) pentaammine chloride catalyzed by colloidal palladium in water/AOT/n-heptane microemulsions. Langmuir 16:2464–2468
Lv FF, Zheng LQ, Tung CH (2005) Phase behavior of the microemulsions and the stability of the chloramphenicol in the microemulsion-based ocular drug delivery system. Int Pharm 301:237–246
Landfester K, Willert M, Antonietti M (2000) Preparation of polymer particles in nonaqueous direct and inverse miniemulsions. Macromolecules 33:2370–2376
Welton T (1999) Room-temperature ionic liquids: solvents for synthesis and catalysis. Chem Rev 99:2071–2084
Mallakpour S, Seyedjamali H (2009) Fast synthesis of optically active polyamides containing L-methionine linkages in ionic liquid via a microwave-assisted process. Colloid Polym Sci 287:1111–1116
Parvulescu VI, Hardacre C (2007) Catalysis in ionic liquids. Chem Rev 107:2615–2665
Haumann M, Riisager A (2008) Hydroformylation in room temperature ionic liquids (RTILs): catalyst and process developments. Chem Rev 108:1474–1497
Cheng N, Yu PM, Wang T, Sheng X, Bi YH, Gong YJ, Yu L (2014) Self-aggregation of new alkylcarboxylate-based anionic surface active ionic liquids: experimental and theoretical investigations. J Phys Chem B 118:2758–276
Fu CP, Zhou HH, Wu HM, Chen JH, Kuang YF (2008) Research on electrochemical properties of nonaqueous ionic liquid microemulsions. Colloid Polym Sci 286:1499–1504
Walden P (1914) Molecular weights and electrical conductivity of several fused salts. Bull Acad Imper Sci (St Petersburg) 8:405–422
Eastoe J, Gold S, Rogers SE, Paul A, Welton T, Heenan RK, Grillo I (2005) Ionic liquid-in-oil microemulsions. J Am Chem Soc 127:7302–7303
Greaves TL, Drummond CJ (2008) Ionic liquids as amphiphile self-assembly media. Chem Soc Rev 37:1709–1726
Qiu Z, Texte J (2008) Ionic liquids in microemulsions. Curr Opin Colloid Interface Sci 13:252–262
Okada Y, Sawada H (2009) Preparation of novel cross-linked fluoroalkyl end-capped cooligomeric nanoparticles-encapsulated cytochrome c in water and ionic liquids. Colloid Polym Sci 287:1359–1363
Kunz W, Zemb T, Harrar A (2012) Using ionic liquids to formulate microemulsions: current state of affairs. Curr Opin Colloid Interface Sci 17:205–211
Huddleston JG, Rogers RD (1998) Room temperature ionic liquids as novel media for ‘clean’ liquid-liquid extraction. Chem Commun 1765–1766. doi:10.1039/A803999B
Dickinson EV, Williams ME, Hendrickson SM, Masui H, Murray RW (2012) Hybrid redox polyether melts based on polyether-tailed counterions. J Am Chem Soc 121:613–616
Anderson JL, Pino V, Hagberg EC, Sheares VV, Armstrong DW (2003) Surfactant solvation effects and micelle formation in ionic liquids. Chem Commun 2444–2445. doi:10.1039/B307516H
Fletcher KA, Pandey S (2004) Surfactant aggregation within room-temperature ionic liquid 1-ethyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide. Langmuir 20:33–36
Patrascu C, Gauffre F, Nallet F, Bordes R, Oberdisse J, De lauth-Viguerie N, Mingotaud C (2006) Micelles in ionic liquids: aggregation behavior of alkyl poly (ethyleneglycol)-ethers in 1-butyl-3-methyl-imidazolium type ionic liquids. Chem Phys Chem 7:99–101
Chakrabarty D, Seth D, Chakratorty A, Sarkar N (2005) Dynamics of solvation and rotational relaxation of coumarin 153 in ionic liquid confined nanometer-sized microemulsions. J Phys Chem B 109:5753–5758
Gao HX, Li JC, Han BX, Chen WN, Zhang JL, Zhang R, Yan DD (2004) Microemulsions with ionic liquid polar domains. Phys Chem Chem Phys 6:2914–2916
Evans DF, Yamauchi A, Roman R, Casassa EZ (1982) Micelle formation in ethylammonium nitrate, a low-melting fused salt. J Colloid Interface Sci 88:89–96
Evans DF, Chen SH, Schriver GW, Arnett EM (1981) Thermodynamics of solution of nonpolar gases in a fused salt. Hydrophobic bonding behavior in a nonaqueous system. J Am Chem Soc 103:481–482
Evans DF, Kaler EW, Benton WJ (1983) Liquid crystals in a fused salt: β, γ-distearoylphosphatidylcholine in N-ethylammonium nitrate. J Phys Chem 87:533–535
Araos MU, Warr GG (2005) Self-assembly of nonionic surfactants into lyotropic liquid crystals in ethylammonium nitrate, a room-temperature ionic liquid. J Phys Chem B 109:14275–14277
Atkin R, Warr GG (2005) Self-assembly of a nonionic surfactant at the graphite/ionic liquid interface. J Am Chem Soc 127:11940–11941
Atkin R, Warr GG (2007) Phase behavior and microstructure of microemulsions with a room-temperature ionic liquid as the polar phase. J Phys Chem B 111:9309–9316
Gao YA, Zhang J, Xu HY, Zhao XY, Zheng LQ, Li XW, Yu L (2006) Structural studies of 1-butyl-3-methylimidazolium tetrafluoroborate/TX-100/ p-xylene ionic liquid microemulsions. Chem Phys Chem 7:1554–1561
Xu J, Zhang L, Yi AL, Hou WG, Yang Y (2013) Nonaqueous ionic liquid microemulsions of 1-butyl-3-methylimidazolium tetrafluoroborate, toluene and ethanol. Soft Matter 9:6497–9504
Mandal S, Ghosh S, Banerjee C, Kuchlyan J, Banik D, Sarkar N (2013) A novel ionic liquid-in-oil microemulsion composed of biologically acceptable components: an excitation wavelength dependent fluorescence resonance energy transfer study. J Phys Chem B 117:3221–3231
Bernot RJ, Kennedy EE, Lamberti GA (2005) Effects of ionic liquids on the survival, movement, and feeding behavior of the freshwater snail, Physa acuta. Environ Toxicol Chem 24:1759–1765
Dai N, Zhang FJ, Chen L, Deng YF, Li S (2009) Research on the biodegradability of IL [Omin][PF6]. Environ Sci 30:480–483
Pretti C, Chiappe C, Pieraccini D, Gregori M, Abramo F, Monni G, Intorre L (2006) Acute toxicity of ionic liquids to the zebrafish (Danio rerio). Green Chem 8:238–240
Clausse M, Zradba A, Nicolas-Morgantini L (1987) In microemulsion systems. Marcel Dekker, New York
Safavi A, Maleki N, Farjami F (2010) Phase behavior and characterization of ionic liquids based microemulsions. Colloids Surf A 355:61–66
Zhu DM, Schelly ZA (1992) Investigation of the microenvironment in Triton X-100 reverse micelles in cyclohexane, using methyl orange as a probe. Langmuir 8:48–50
Acknowledgments
This work was supported by the National Science Fund for Distinguished Young Scholars (51425406), the National Natural Science Foundation of China (51174221, 21303268), the Doctoral Fund from National Ministry of Education (20120133110010), and the China Postdoctoral Science Foundation Fund Project (2013T60689).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, C., Wang, S., Du, M. et al. Phase behavior of a nonaqueous ternary microemulsion containing ethylammonium nitrate, TX-100, and cyclohexane. Colloid Polym Sci 293, 1475–1481 (2015). https://doi.org/10.1007/s00396-015-3525-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3525-8