Abstract
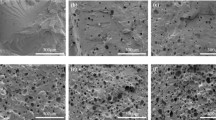
Here, we present a method to synthesize expandable spherical polystyrene beads containing well-dispersed water microdroplets. The beads, 2–3 mm in diameter, were prepared through surfactant-free Pickering emulsion polymerization in water-in-oil-in-water (w/o/w) system using cross-linked starch nanoparticles (CSTN) as emulsifier. The CSTNs were in situ surface-modified by styrene maleic anhydride copolymer as confirmed by infrared spectroscopy and contact angle analysis. The entrapped water microdroplets with the average size of 3–4 μm were shown to be surrounded by a dense layer of the CSTN. The number droplet density as well as water encapsulation efficiency in the polystyrene beads increased with the CSTN concentration. Furthermore, regardless of CSTN content, all samples exhibited high encapsulation stability of over 68 % after 3 months. These characteristics along with good expansion behavior suggest the synthesized beads as expandable polystyrene containing water as a green blowing agent.












Similar content being viewed by others
References
Pickering SU (1907) Journal of the Chemical Society, Transactions 91
Tambe DE, Sharma MM (1994) Factors controlling the stability of colloid-stabilized emulsions: II. A model for the rheological properties of colloid-laden interfaces. J Colloid Interface Sci 162(1):1–10. doi:10.1006/jcis.1994.1001
Tambe DE, Sharma MM (1995) Factors controlling the stability of colloid-stabilized emulsions: III. Measurement of the rheological properties of colloid-laden interfaces. J Colloid Interface Sci 171(2):456–462. doi:10.1006/jcis.1995.1202
Midmore BR (1998) Preparation of a novel silica-stabilized oil/water emulsion. Colloids Surf A Physicochem Eng Asp 132(2–3):257–265. doi:10.1016/S0927-7757(97)00094-0
Horozov TS, Binks BP (2006) Particle-stabilized emulsions: A bilayer or a bridging monolayer? Angew Chem Int Ed 45(5):773–776. doi:10.1002/anie.200503131
Marku D, Wahlgren M, Rayner M, Sjöö M, Timgren A (2012) Characterization of starch Pickering emulsions for potential applications in topical formulations. Int J Pharm 428(1–2):1–7. doi:10.1016/j.ijpharm.2012.01.031
Yusoff A, Murray BS (2011) Modified starch granules as particle-stabilizers of oil-in-water emulsions. Food Hydrocoll 25(1):42–55. doi:10.1016/j.foodhyd.2010.05.004
Dokić L, Krstonošić V, Nikolić I (2012) Physicochemical characteristics and stability of oil-in-water emulsions stabilized by OSA starch. Food Hydrocoll 29(1):185–192. doi:10.1016/j.foodhyd.2012.02.008
Rayner M, Sjoo M, Timgren A, Dejmek P (2012) Quinoa starch granules as stabilizing particles for production of Pickering emulsions. Faraday Discuss 158:139–155. doi:10.1039/c2fd20038d
Timgren A, Rayner M, Sjöö M, Dejmek P (2011) Starch particles for food based Pickering emulsions. Procedia Food Sci 1(0):95–103. doi:10.1016/j.profoo.2011.09.016
Matos M, Timgren A, Sjöö M, Dejmek P, Rayner M (2013) Preparation and encapsulation properties of double Pickering emulsions stabilized by quinoa starch granules. Colloids Surf A Physicochem Eng Asp 423(0):147–153. doi:10.1016/j.colsurfa.2013.01.060
Li C, Sun P, Yang C (2012) Emulsion stabilized by starch nanocrystals. Starch Stärke 64(6):497–502. doi:10.1002/star.201100178
Tan Y, Xu K, Liu C, Li Y, Lu C, Wang P (2012) Fabrication of starch-based nanospheres to stabilize pickering emulsion. Carbohydr Polym 88(4):1358–1363. doi:10.1016/j.carbpol.2012.02.018
Xu ZZ, Wang CC, Yang WL, Deng YH, Fu SK (2004) Encapsulation of nanosized magnetic iron oxide by polyacrylamide via inverse miniemulsion polymerization. J Magn Magn Mater 277(1–2):136–143. doi:10.1016/j.jmmm.2003.10.018
Ge L, Texter J (2004) Combustion resistant nanocomposites from water/AOT/MMA reverse microemulsions. Polym Bull 52(3–4):297–305. doi:10.1007/s00289-004-0288-7
Negrete-Herrera N, Putaux J-L, David L, Bourgeat-Lami E (2006) Polymer/Laponite composite colloids through emulsion polymerization: Influence of the clay modification level on particle morphology. Macromolecules 39(26):9177–9184. doi:10.1021/ma0610515
Zhang K, Wu W, Meng H, Guo K, Chen JF (2009) Pickering emulsion polymerization: preparation of polystyrene/nano-SiO2 composite microspheres with core–shell structure. Powder Technol 190(3):393–400. doi:10.1016/j.powtec.2008.08.022
Voorn DJ, Ming W, van Herk AM (2006) Polymer–clay nanocomposite latex particles by inverse Pickering emulsion polymerization stabilized with hydrophobic montmorillonite platelets. Macromolecules 39(6):2137–2143. doi:10.1021/ma052539t
Fang FF, Kim JH, Choi HJ, Kim CA (2009) Synthesis and electrorheological response of nanosized Laponite stabilized poly(methyl methacrylate) spheres. Colloid Polym Sci 287(6):745–749
Liu YD, Zhang WL, Choi HJ (2012) Pickering emulsion polymerization of core-shell-structured polyaniline@SiO 2 nanoparticles and their electrorheological response. Colloid Polym Sci 290(9):855–860
Crevecoeur JJ, Nelissen L, Lemstra PJ (1999) Water expandable polystyrene (WEPS): Part 1. Strategy and procedures. Polymer 40(13):3685–3689. doi:10.1016/S0032-3861(98)00617-X
Snijders Emile A, Nelissen L, Lemstra Piet J (2006) Water expandable polystyrene (WEPS): Part 4. Synthesis of the water expandable blend of polystyrene and poly(2,6- dimethyl-1,4-phenylene ether). e-Polymers, vol 6. doi:10.1515/epoly.2006.6.1.994
Shen J, Cao X, James Lee L (2006) Synthesis and foaming of water expandable polystyrene–clay nanocomposites. Polymer 47(18):6303–6310. doi:10.1016/j.polymer.2006.06.068
Crevecoeur JJ, Nelissen L, Lemstra PJ (1999) Water expandable polystyrene (WEPS): Part 2. In situ synthesis of (block) copolymer surfactants. Polymer 40(13):3691–3696. doi:10.1016/S0032-3861(98)00619-3
Pallay J, Kelemen P, Berghmans H, Van Dommelen D (2000) Expansion of polystyrene using water as the blowing agent. Macromol Mater Eng 275(1):18–25. doi:10.1002/(sici)1439-2054(20000201)275:1<18::aid-mame18>3.0.co;2-3
Amiri R-SN, Qazvini NT, Sanjani NS (2009) Water expandable polystyrene-organoclay nanocomposites: Role of clay and its dispersion state. J Macromol Sci B 48(5):955–966. doi:10.1080/00222340903032474
Garti N (1997) Progress in stabilization and transport phenomena of double emulsions in food applications. LWT Food Sci Technol 30(3):222–235. doi:10.1006/fstl.1996.0176
Ma X, Jian R, Chang PR, Yu J (2008) Fabrication and characterization of citric acid-modified starch nanoparticles/plasticized-starch composites. Biomacromolecules 9(11):3314–3320. doi:10.1021/bm800987c
Fang JM, Fowler PA, Tomkinson J, Hill CAS (2002) The preparation and characterisation of a series of chemically modified potato starches. Carbohydr Polym 47(3):245–252. doi:10.1016/S0144-8617(01)00187-4
Putaux J-L, Molina-Boisseau S, Momaur T, Dufresne A (2003) Platelet nanocrystals resulting from the disruption of waxy maize starch granules by acid hydrolysis. Biomacromolecules 4(5):1198–1202. doi:10.1021/bm0340422
Huang M-F, Yu J-G, Ma X-F (2004) Studies on the properties of Montmorillonite-reinforced thermoplastic starch composites. Polymer 45(20):7017–7023. doi:10.1016/j.polymer.2004.07.068
van Soest JJG, Vliegenthart JFG (1997) Crystallinity in starch plastics: consequences for material properties. Trends Biotechnol 15(6):208–213. doi:10.1016/S0167-7799(97)01021-4
Ma X, Yu J, Wang N (2008) Glycerol plasticized-starch/multiwall carbon nanotube composites for electroactive polymers. Compos Sci Technol 68(1):268–273. doi:10.1016/j.compscitech.2007.03.016
Zobel HF, Young SN, Rocca LA (1988) Starch gelatinization: An X-ray diffraction study. Cereal Chem 65(6):4
Xie X, Liu Q, Cui SW (2006) Studies on the granular structure of resistant starches (type 4) from normal, high amylose, and waxy cornstarch citrates. Food Res Int 39(3):332–341. doi:10.1016/j.foodres.2005.08.004
Pallay J, Berghmans H (2002) Water-blown expandable polystyrene. Improvement of the compatibility of the water carrier with the polystyrene matrix by In Situ grafting Part I. Mechanism of free radical grafting. Cell Polym 21(1):1–18
Aveyard R, Binks BP, Clint JH (2003) Emulsions stabilized solely by colloidal particles. Adv Colloid Interf Sci 100–102(SUPPL):503–546
Andresen M, Stenius P (2007) Water-in-oil emulsions stabilized by hydrophobized microfibrillated cellulose. J Dispers Sci Technol 28(6):837–844
Vaidya UR, Bhattacharya M (1994) Properties of blends of starch and synthetic polymers containing anhydride groups. J Appl Polym Sci 52(5):617–628. doi:10.1002/app.1994.070520505
Chauhan GS, Guleria LK, Misra BN, Kaur I (1999) Polymers from renewable resources. II. A study in the radio chemical grafting of poly(styrene-alt-maleic anhydride) onto cellulose extracted from pine needles. J Polym Sci A Polym Chem 37(12):1763–1769. doi:10.1002/(sici)1099-0518(19990615)37:12<1763::aid-pola5>3.0.co;2-s
Bastos DC, Santos AEF, da Silva MLVJ, Simão RA (2009) Hydrophobic cornstarch thermoplastic films produced by plasma treatment. Ultramicroscopy 109(8):1089–1093. doi:10.1016/j.ultramic.2009.03.031
Midmore BR (1999) Effect of aqueous phase composition on the properties of a silica-stabilized w/o emulsion. J Colloid Interface Sci 213(2):352–359. doi:10.1006/jcis.1999.6108
Binks BP, Lumsdon SO (2000) Catastrophic phase inversion of water-in-oil emulsions stabilized by hydrophobic silica. Langmuir 16(6):2539–2547. doi:10.1021/la991081j
Binks BP, Whitby CP (2004) Silica particle-stabilized emulsions of silicone oil and water: aspects of emulsification. Langmuir 20(4):1130–1137. doi:10.1021/la0303557
Binks BP, Philip J, Rodrigues JA (2005) Inversion of silica-stabilized emulsions induced by particle concentration. Langmuir 21(8):3296–3302. doi:10.1021/la046915z
Abismaı̈l B, Canselier JP, Wilhelm AM, Delmas H, Gourdon C (1999) Emulsification by ultrasound: drop size distribution and stability. Ultrason Sonochem 6(1–2):75–83. doi:10.1016/S1350-4177(98)00027-3
Behrend O, Ax K, Schubert H (2000) Influence of continuous phase viscosity on emulsification by ultrasound. Ultrason Sonochem 7(2):77–85. doi:10.1016/S1350-4177(99)00029-2
Das AK, Mukesh D, Swayambunathan V, Kotkar DD, Ghosh PK (1992) Concentrated emulsions. 3. Studies on the influence of continuous-phase viscosity, volume fraction, droplet size, and temperature on emulsion viscosity. Langmuir 8(10):2427–2436
Huang X, Kakuda Y, Cui W (2001) Hydrocolloids in emulsions: particle size distribution and interfacial activity. Food Hydrocoll 15(4–6):533–542. doi:10.1016/S0268-005X(01)00091-1
Yan N, Masliyah JH (1995) Characterization and demulsification of solids-stabilized oil-in-water emulsions Part 1. Partitioning of clay particles and preparation of emulsions. Colloids Surf A Physicochem Eng Asp 96(3):229–242. doi:10.1016/0927-7757(94)03058-8
Binks BP, Clint JH, Dyab AKF, Fletcher PDI, Kirkland M, Whitby CP (2003) Ellipsometric study of monodisperse silica particles at an oil–water interface. Langmuir 19(21):8888–8893. doi:10.1021/la035058g
Raghavan SR, Walls HJ, Khan SA (2000) Rheology of silica dispersions in organic liquids: new evidence for solvation forces dictated by hydrogen bonding. Langmuir 16(21):7920–7930. doi:10.1021/la991548q
Raghavan SR, Khan SA (1995) Shear-induced microstructural changes in flocculated suspensions of fumed silica. J Rheol 39(6):1311–1325
Acknowledgment
The authors thank the Institute of Paper Science and Technology (IPST), Georgia Tech for experimental facilities and infrastructure. Rui Zhao, Sudhier Sharma, and Wei Mu in IPST are greatly acknowledged for providing technical support. We also thank the respected reviewers for their helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding authors
Appendix A: calculation of n pt /n pa ratio
Appendix A: calculation of n pt /n pa ratio
The total number of CSTN particle in a defined volume of bead can be calculated from:
For water droplets of radius r e stabilized by CSTN particles of radius r p , assuming that r e > r p and that the particles are arranged in a hexagonal close-packed (HCP) arrangement on droplet surfaces and taking the contact angle that the particles make with the oil–water interface equal to 90°, the total number of particles surrounding all droplets in a defined volume of bead (V b ) is as follows:
Combination of equations A1–A2 leads to the following expression for the total number of particles available to the number of particles required to form a single layer covering the available droplet surfaces.
With C = weight percent of particle (percent), ρ b = density of bead (grams per cubic meter), ρ p = density of particle (grams per cubic meter), r p = radius of particle (nanometers), r e = radius of droplet (nanometers), and N ∘ = number density of droplets (number per cubic millimeter).
Appendix B: calculation of the number of particle layers around the droplets
The following expression is used to find an equation to calculate the thickness of particle layers (x) around the droplets in a defined volume of bead and taking the contact angle that the particles make with the oil–water interface equal to 90°:
This yields

With C = weight percent of particle (percent), ρ b = density of bead (grams per cubic centimeter), ρ p = density of particle (grams per cubic centimeter), r e = radius of droplet (nanometers), N ∘ = number density of droplets (numbers per cubic millimeter), and x = thickness of particle layer (nanometers).
With considering the HCP unit cell height and the number of layers in every unit cell, the number of layers around the droplets (L) can be calculated by the following equation:
Rights and permissions
About this article
Cite this article
Nikfarjam, N., Taheri Qazvini, N. & Deng, Y. Cross-linked starch nanoparticles stabilized Pickering emulsion polymerization of styrene in w/o/w system. Colloid Polym Sci 292, 599–612 (2014). https://doi.org/10.1007/s00396-013-3102-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-013-3102-y