Abstract
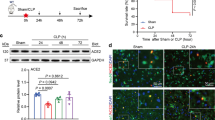
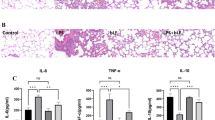
The NLRP3 inflammasome is an intracellular multiple-protein complex that controls the maturation and release of interleukin (IL)-1β and IL-18. Endogenous carbon monoxide (CO) is anti-inflammatory. The aim of this study was to assess the effects/mechanisms of CO-releasing molecule-3 (CORM-3)-dependent modulation of the NLRP3 inflammasome in cardiac fibroblasts (CF) and its effect on myocardial function in sepsis. CF were treated with CORM-3 or inactive CORM-3 (iCORM-3) before NLRP3 inflammasome priming with lipopolysaccharides (LPS) or following activation with adenosine triphosphate (ATP). In parallel, cardiomyocytes (CM) were challenged with supernatants of LPS/ATP-stimulated CF or a cytokine mixture (Cyto-mix) containing IL-1β, IL-18, and HMGB1. In vivo, mice were treated with CORM-3 before or after LPS to induce sepsis (endotoxemia). Pretreatment of CF with CORM-3 prevented an LPS-induced increase in NLRP3 and pro-IL-1β expression. Treatment of CF with CORM-3 before ATP prevented ATP-induced activation of the NLRP3 inflammasome. Challenging CF with LPS/ATP promoted NLRP3 interactions with adaptor ASC (apoptosis-associated speck-like protein containing a caspase-recruitment domain), which was prevented by CORM-3. Challenging CM with supernatants of CF with LPS/ATP or Cyto-mix (IL-1β, IL-18, and HMGB1) resulted in CM apoptosis, which was attenuated with either a CORM-3 or IL-1 receptor antagonist. Finally, myocardial NLRP3 inflammasome activation and myocardial dysfunction in septic mice were abolished by CORM-3. In NLRP3-deficient mice with sepsis, CORM-3 did not show additional benefits in improving myocardial function. Our results indicate that CORM-3 suppresses NLRP3 inflammasome activation by blocking NLRP3 interactions with the adaptor protein ASC and attenuates myocardial dysfunction in mice with sepsis.








Similar content being viewed by others
References
Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graca-Souza AV, Ollinger R, Czismadia E, May GA, Ifedigbo E, Otterbein LE, Bach FH, Soares MP (2004) Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J 18:771–772. doi:10.1096/fj.03-0921fje
An R, Zhao L, Xi C, Li H, Shen G, Liu H, Zhang S, Sun L (2016) Melatonin attenuates sepsis-induced cardiac dysfunction via a PI3K/Akt-dependent mechanism. Basic Res Cardiol 111:8. doi:10.1007/s00395-015-0526-1
Angus DC, van der Poll T (2013) Severe sepsis and septic shock. N Engl J Med 369:840–851. doi:10.1056/NEJMc1312359
Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365:63–78. doi:10.1016/S0140-6736(04)17667-8
Bracey NA, Gershkovich B, Chun J, Vilaysane A, Meijndert HC, Wright JR Jr, Fedak PW, Beck PL, Muruve DA, Duff HJ (2014) Mitochondrial NLRP3 protein induces reactive oxygen species to promote Smad protein signaling and fibrosis independent from the inflammasome. J Biol Chem 289:19571–19584. doi:10.1074/jbc.M114.550624
Cohen J (2002) The immunopathogenesis of sepsis. Nature 420:885–891. doi:10.1038/nature01326
Czibik G, Derumeaux G, Sawaki D, Valen G, Motterlini R (2014) Heme oxygenase-1: an emerging therapeutic target to curb cardiac pathology. Basic Res Cardiol 109:450. doi:10.1007/s00395-014-0450-9
Dong M, Hu N, Hua Y, Xu X, Kandadi MR, Guo R, Jiang S, Nair S, Hu D, Ren J (2013) Chronic Akt activation attenuated lipopolysaccharide-induced cardiac dysfunction via Akt/GSK3beta-dependent inhibition of apoptosis and ER stress. Biochim Biophys Acta 1832:848–863. doi:10.1016/j.bbadis.2013.02.023
Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J, Wang Y, Zingarelli B, Peng T, Fan GC (2015) Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta 1852:2362–2371. doi:10.1016/j.bbadis.2015.08.010
Fallach R, Shainberg A, Avlas O, Fainblut M, Chepurko Y, Porat E, Hochhauser E (2010) Cardiomyocyte Toll-like receptor 4 is involved in heart dysfunction following septic shock or myocardial ischemia. J Mol Cell Cardiol 48:1236–1244. doi:10.1016/j.yjmcc.2010.02.020
Guo H, Callaway JB, Ting JP (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687. doi:10.1038/nm.3893
Haneklaus M, O’Neill LA (2015) NLRP3 at the interface of metabolism and inflammation. Immunol Rev 265:53–62. doi:10.1111/imr.12285
Ichinose F, Buys ES, Neilan TG, Furutani EM, Morgan JG, Jassal DS, Graveline AR, Searles RJ, Lim CC, Kaneki M, Picard MH, Scherrer-Crosbie M, Janssens S, Liao R, Bloch KD (2007) Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circ Res 100:130–139. doi:10.1161/01.RES.0000253888.09574.7a
Jo EK, Kim JK, Shin DM, Sasakawa C (2016) Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13:148–159. doi:10.1038/cmi.2015.95
Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U (2011) Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123:594–604. doi:10.1161/CIRCULATIONAHA.110.982777
Kim SK, Joe Y, Chen Y, Ryu J, Lee JH, Cho GJ, Ryter SW, Chung HT (2015) Carbon monoxide decreases interleukin-1beta levels in the lung through the induction of pyrin. Cell Mol Immunol. doi:10.1038/cmi.2015.79
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13:397–411. doi:10.1038/nri3452
Liu Y, Lian K, Zhang L, Wang R, Yi F, Gao C, Xin C, Zhu D, Li Y, Yan W, Xiong L, Gao E, Wang H, Tao L (2014) TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol 109:415. doi:10.1007/s00395-014-0415-z
Matsuno K, Iwata K, Matsumoto M, Katsuyama M, Cui W, Murata A, Nakamura H, Ibi M, Ikami K, Zhang J, Matoba S, Jin D, Takai S, Matsubara H, Matsuda N, Yabe-Nishimura C (2012) NOX1/NADPH oxidase is involved in endotoxin-induced cardiomyocyte apoptosis. Free Radic Biol Med 53:1718–1728. doi:10.1016/j.freeradbiomed.2012.08.590
Musameh MD, Green CJ, Mann BE, Motterlini R, Fuller BJ (2010) CO liberated from a carbon monoxide-releasing molecule exerts a positive inotropic effect in doxorubicin-induced cardiomyopathy. J Cardiovasc Pharmacol 55:168–175. doi:10.1097/FJC.0b013e3181ca4bbc
Ni R, Cao T, Xiong S, Ma J, Fan GC, Lacefield JC, Lu Y, Tissier SL, Peng T (2016) Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic Biol Med 90:12–23. doi:10.1016/j.freeradbiomed.2015.11.013
Patterson EK, Fraser DD, Capretta A, Potter RF, Cepinskas G (2014) Carbon monoxide-releasing molecule 3 inhibits myeloperoxidase (MPO) and protects against MPO-induced vascular endothelial cell activation/dysfunction. Free Radic Biol Med 70:167–173. doi:10.1016/j.freeradbiomed.2014.02.020
Piek A, de Boer RA, Sillje HH (2016) The fibrosis-cell death axis in heart failure. Heart Fail Rev 21:199–211. doi:10.1007/s10741-016-9536-9
Rathinam VA, Vanaja SK, Fitzgerald KA (2012) Regulation of inflammasome signaling. Nat Immunol 13:333–342. doi:10.1038/ni.2237
Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ (2011) Sepsis-induced cardiomyopathy. Curr. Cardiol Rev 7:163–183. doi:10.2174/157340311798220494
Rubartelli A (2012) Redox control of NLRP3 inflammasome activation in health and disease. J Leukoc Biol 92:951–958. doi:10.1189/jlb.0512265
Rui T, Tang Q (2013) IL-33 attenuates anoxia/reoxygenation-induced cardiomyocyte apoptosis by inhibition of PKCbeta/JNK pathway. PLoS One 8:e56089. doi:10.1371/journal.pone.0056089
Rui T, Zhang J, Xu X, Yao Y, Kao R, Martin CM (2012) Reduction in IL-33 expression exaggerates ischaemia/reperfusion-induced myocardial injury in mice with diabetes mellitus. Cardiovasc Res 94:370–378. doi:10.1093/cvr/cvs015
Sandanger O, Ranheim T, Vinge LE, Bliksoen M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G, Christensen G, Fitzgerald KA, Lien E, Valen G, Espevik T, Aukrust P, Yndestad A (2013) The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res 99:164–174. doi:10.1093/cvr/cvt091
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140:821–832. doi:10.1016/j.cell.2010.01.040
Shen L, Yang S, Yang T, Liang J, Cheng W, Wen J, Liu Y, Li J, Shi L, Tang Q, Shi W, Hu J, Liu C, Zhang Y, Mou S, Liu Z, Cai H, He L, Guan D, Wu Y, He S (2016) CaCDPK15 positively regulates pepper responses to Ralstonia solanacearum inoculation and forms a positive-feedback loop with CaWRKY40 to amplify defense signaling. Sci Rep 6:22439. doi:10.1038/srep22439
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810. doi:10.1001/jama.2016.0287
Tao A, Song J, Lan T, Xu X, Kvietys P, Kao R, Martin C, Rui T (2015) Cardiomyocyte-fibroblast interaction contributes to diabetic cardiomyopathy in mice: role of HMGB1/TLR4/IL-33 axis. Biochim Biophys Acta 1852:2075–2085. doi:10.1016/j.bbadis.2015.07.015
Ulland TK, Ferguson PJ, Sutterwala FS (2015) Evasion of inflammasome activation by microbial pathogens. J Clin Invest 125:469–477. doi:10.1172/JCI75254
Wang X, Zingarelli B, O’Connor M, Zhang P, Adeyemo A, Kranias EG, Wang Y, Fan GC (2009) Overexpression of Hsp20 prevents endotoxin-induced myocardial dysfunction and apoptosis via inhibition of NF-kappaB activation. J Mol Cell Cardiol 47:382–390. doi:10.1016/j.yjmcc.2009.05.016
Wu L, Wang R (2005) Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev 57:585–630. doi:10.1124/pr.57.4.3
Xu H, Su Z, Wu J, Yang M, Penninger JM, Martin CM, Kvietys PR, Rui T (2010) The alarmin cytokine, high mobility group box 1, is produced by viable cardiomyocytes and mediates the lipopolysaccharide-induced myocardial dysfunction via a TLR4/phosphatidylinositol 3-kinase gamma pathway. J Immunol 184:1492–1498. doi:10.4049/jimmunol.0902660
Xu H, Yao Y, Su Z, Yang Y, Kao R, Martin CM, Rui T (2011) Endogenous HMGB1 contributes to ischemia/reperfusion-induced myocardial apoptosis by potentiating the effect of TNF{alpha}/JNK. Am J Physiol Heart Circ Physiol 300:H913–H921. doi:10.1152/ajpheart.00703.2010
Yao Y, Xu X, Zhang G, Zhang Y, Qian W, Rui T (2012) Role of HMGB1 in doxorubicin-induced myocardial apoptosis and its regulation pathway. Basic Res Cardiol 107:267. doi:10.1007/s00395-012-0267-3
Zhang W, Xu X, Kao R, Mele T, Kvietys P, Martin CM, Rui T (2014) Cardiac fibroblasts contribute to myocardial dysfunction in mice with sepsis: the role of NLRP3 inflammasome activation. PLoS One 9:e107639. doi:10.1371/journal.pone.0107639
Acknowledgements
We thank Ms. Xuemei Xu for performing mouse myocardial function measurement. This work was supported by Grants from Lawson Health Research Institute IRF 2014-25, National Natural Science Foundation of China 81370333, Natural Science Foundation of Jiangsu Province, China BK2015-1332 and by the “Six Talent Peaks” Training Program, Jiangsu, China WSN-079.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
W. Zhang and A. Tao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, W., Tao, A., Lan, T. et al. Carbon monoxide releasing molecule-3 improves myocardial function in mice with sepsis by inhibiting NLRP3 inflammasome activation in cardiac fibroblasts. Basic Res Cardiol 112, 16 (2017). https://doi.org/10.1007/s00395-017-0603-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-017-0603-8