Abstract
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor that contributes a crucial role in protection against ischemia (ISC)-reperfusion (REP) injury by driving expression of anti-apoptotic and anti-oxidant genes. STAT3 is also present in the mitochondria, where it modulates the activity of the electron transport chain (ETC) and the permeability transition pore. Transgenic mice that overexpress a mitochondrial-targeted, transcriptionally inactive STAT3 in cardiomyocytes (MLS-STAT3E mice) exhibit a persistent, partial blockade of electron transfer through complex I that uniquely did not lead to tissue dysfunction at baseline, yet increased mitochondrial ischemic tolerance. The direct contribution of non-transcriptional, mitochondria-localized STAT3 to protection during ISC-REP remains to be established. We hypothesized that the enhanced mitochondrial tolerance to ischemia present in MLS-STAT3E mice would decrease cardiac injury during ISC-REP. In the isolated buffer-perfused heart model, MLS-STAT3E hearts exhibit a decreased infarct size compared to non-transgenic littermate hearts. Contractile recovery, expressed as a percent of LV developed pressure before ISC, is improved in MLS-STAT3E mice. Mitochondria isolated at the end of 60 min. of REP from MLS-STAT3E hearts show attenuated ROS release. The partial and persistent blockade of complex I present in MLS-STAT3E mice decreases cardiac injury during REP, in part via a persistent decrease in ROS production and attenuation of mitochondrial permeability transition pore opening at the onset of REP. In vivo, MLS-STAT3E hearts exhibit substantially higher postoperative survival rate and a substantial decrease in myocardial infarct size. STAT3 mediates cardioprotection not only via canonical action as a transcription factor, but also as a modulator of ETC activity directly in the mitochondria.





Similar content being viewed by others
References
Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK (2008) Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res 77:406–415. doi:10.1016/j.cardiores.2007.08.008
Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R (2008) Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res 102:131–135. doi:10.1161/CIRCRESAHA.107.164699
Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R (2008) The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther 120:172–185. doi:10.1016/j.pharmthera.2008.08.002
Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R (2010) Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol 105:771–785. doi:10.1007/s00395-010-0124-1
Boengler K, Ungefug E, Heusch G, Schulz R (2013) The STAT3 inhibitor stattic impairs cardiomyocyte mitochondrial function through increased reactive oxygen species formation. Curr Pharm Des 19:6890–6895. doi:10.2174/138161281939131127115940
Borutaite V, Brown GC (2003) Mitochondria in apoptosis of ischemic heart. FEBS Lett 541:1–5. doi:10.1016/S0014-5793(03)00278-3
Borutaite V, Budriunaite A, Morkuniene R, Brown GC (2001) Release of mitochondrial cytochrome c and activation of cytosolic caspases induced by myocardial ischaemia. Biochim Biophys Acta 1537:101–109. doi:10.1016/S0925-4439(01)00062-X
Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ (2006) Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther 319:1405–1412. doi:10.1124/jpet.106.110262
Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ (2008) Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol 294:C460–C466. doi:10.1152/ajpcell.00211.2007
Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ (2003) Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278:36027–36031. doi:10.1074/jbc.M304854200
Cohen MV, Yang XM, Downey JM (2007) The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation 115:1895–1903. doi:10.1161/CIRCULATIONAHA.106.675710
Crompton M (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem J 341:233–249. doi:10.1016/S0022-2828(03)00043-9
Darnell JE Jr (1997) STATs and gene regulation. Science 277:1630–1635. doi:10.1126/science.277.5332.1630
Doetschman T (2009) Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol Biol 530:423–433. doi:10.1007/978-1-59745-471-1_23
Fuglesteg BN, Suleman N, Tiron C, Kanhema T, Lacerda L, Andreasen TV, Sack MN, Jonassen AK, Mjos OD, Opie LH, Lecour S (2008) Signal transducer and activator of transcription 3 is involved in the cardioprotective signalling pathway activated by insulin therapy at reperfusion. Basic Res Cardiol 103:444–453. doi:10.1007/s00395-008-0728-x
Guo Y, Flaherty MP, Wu WJ, Tan W, Zhu X, Li Q, Bolli R (2012) Genetic background, gender, age, body temperature, and arterial blood pH have a major impact on myocardial infarct size in the mouse and need to be carefully measured and/or taken into account: results of a comprehensive analysis of determinants of infarct size in 1074 mice. Basic Res Cardiol 107:288. doi:10.1007/s00395-012-0288-y
Halestrap AP, Clarke SJ, Javadov SA (2004) Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res 61:372–385. doi:10.1016/S0008-6363(03)00533-9
Halestrap AP, Connern CP, Griffiths EJ, Kerr PM (1997) Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem 174:167–172. doi:10.1023/A:1006879618176
Hausenloy DJ, Ong SB, Yellon DM (2009) The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res Cardiol 104:189–202. doi:10.1007/s00395-009-0010-x
Hedayati N, Schomisch SJ, Carino JL, Timothy Sherwood J, Lesnefsky EJ, Cmolik BL (2003) Cardioprotection by St Thomas’ solution is mediated by protein kinase C and tyrosine kinase. J Surg Res 113:121–127. doi:10.1016/S0022-4804(03)00146-X
Heusch G, Musiolik J, Gedik N, Skyschally A (2011) Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res 109:1302–1308. doi:10.1161/CIRCRESAHA.111.255604
Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H (2007) A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128:589–600. doi:10.1016/j.cell.2006.12.036
Lecour S, Suleman N, Deuchar GA, Somers S, Lacerda L, Huisamen B, Opie LH (2005) Pharmacological preconditioning with tumor necrosis factor-alpha activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase). Circulation 112:3911–3918. doi:10.1161/CIRCULATIONAHA.105.581058
Lee Y, Lee HY, Gustafsson AB (2012) Regulation of autophagy by metabolic and stress signaling pathways in the heart. J Cardiovasc Pharmacol 60:118–124. doi:10.1097/FJC.0b013e318256cdd0
Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL (1997) Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol 273:H1544–H1554
Levy DE, Lee CK (2002) What does Stat3 do? J Clin Invest 109:1143–1148. doi:10.1172/JCI15650
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marques SM, Campos PP, Castro PR, Cardoso CC, Ferreira MA, Andrade SP (2011) Genetic background determines mouse strain differences in inflammatory angiogenesis. Microvasc Res 82:246–252. doi:10.1016/j.mvr.2011.08.011
Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100:914–922. doi:10.1161/01.RES.0000261924.76669.36
Nadtochiy SM, Burwell LS, Ingraham CA, Spencer CM, Friedman AE, Pinkert CA, Brookes PS (2009) In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol 46:960–968. doi:10.1016/j.yjmcc.2009.01.012
Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183:795–803. doi:10.1083/jcb.200809125
Natarajan SK, Becker DF (2012) Role of apoptosis-inducing factor, proline dehydrogenase, and NADPH oxidase in apoptosis and oxidative stress. Cell Health Cytoskelet 2012:11–27. doi:10.2147/CHC.S4955
Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, Osugi T, Izumi M, Oshima Y, Nakaoka Y, Hirota H, Kishimoto T, Yamauchi-Takihara K (2001) Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation 104:979–981. doi:10.1161/hc3401.095947
Oshima Y, Fujio Y, Nakanishi T, Itoh N, Yamamoto Y, Negoro S, Tanaka K, Kishimoto T, Kawase I, Azuma J (2005) STAT3 mediates cardioprotection against ischemia/reperfusion injury through metallothionein induction in the heart. Cardiovasc Res 65:428–435. doi:10.1016/j.cardiores.2004.10.021
Paillard M, Gomez L, Augeul L, Loufouat J, Lesnefsky EJ, Ovize M (2009) Postconditioning inhibits mPTP opening independent of oxidative phosphorylation and membrane potential. J Mol Cell Cardiol 46:902–909. doi:10.1016/j.yjmcc.2009.02.017
Sarafian TA, Montes C, Imura T, Qi J, Coppola G, Geschwind DH, Sofroniew MV (2010) Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS One 5:e9532. doi:10.1371/journal.pone.0009532
Smith CC, Dixon RA, Wynne AM, Theodorou L, Ong SG, Subrayan S, Davidson SM, Hausenloy DJ, Yellon DM (2010) Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol 299:H1265–H1270. doi:10.1152/ajpheart.00092.2010
Smith RM, Suleman N, Lacerda L, Opie LH, Akira S, Chien KR, Sack MN (2004) Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc Res 63:611–616. doi:10.1016/j.cardiores.2004.06.019
Stewart S, Lesnefsky EJ, Chen Q (2009) Reversible blockade of electron transport with amobarbital at the onset of reperfusion attenuates cardiac injury. Transl Res 153:224–231. doi:10.1016/j.trsl.2009.02.003
Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, Cichy J, Kukreja RC, Dulak J, Lesnefsky EJ, Larner AC (2011) Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem 286:29610–29620. doi:10.1074/jbc.M111.226209
Toldo S, Das A, Mezzaroma E, Chau VQ, Marchetti C, Durrant D, Samidurai A, Van Tassell BW, Yin C, Ockaili RA, Vigneshwar N, Mukhopadhyay ND, Kukreja RC, Abbate A, Salloum FN (2014) Induction of microRNA-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circulation Cardiovasc Genet 7:311–320. doi:10.1161/CIRCGENETICS.113.000381
Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, Moh A, Moghaddas S, Chen Q, Bobbili S, Cichy J, Dulak J, Baker DP, Wolfman A, Stuehr D, Hassan MO, Fu XY, Avadhani N, Drake JI, Fawcett P, Lesnefsky EJ, Larner AC (2009) Function of mitochondrial Stat3 in cellular respiration. Science 323:793–797. doi:10.1126/science.1164551
Xu A, Szczepanek K, Maceyka MW, Ross T, Bowler E, Hu Y, Kenny B, Mehfoud C, Desai PN, Baumgarten CM, Chen Q, Lesnefsky EJ (2014) Transient complex I inhibition at the onset of reperfusion by extracellular acidification decreases cardiac injury. Am J Physiol Cell Physiol 306:C1142–C1153. doi:10.1152/ajpcell.00241.2013
Zhang Q, Raje V, Yakovlev VA, Yacoub A, Szczepanek K, Meier J, Derecka M, Chen Q, Hu Y, Sisler J, Hamed H, Lesnefsky EJ, Valerie K, Dent P, Larner AC (2013) Mitochondrial localized Stat3 promotes breast cancer growth via phosphorylation of serine 727. J Biol Chem 288:31280–31288. doi:10.1074/jbc.M113.505057
Acknowledgments
This work was supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (to E.J.L.), the American Heart Association Postdoctoral Fellowship Award (to K.S.), the American Heart Association Scientist Development Grant (to Q.C.), American Heart Association Scientist Development Grant and Grant in Aid (to F.N.S.) and the Pauley Heart Center, Virginia Commonwealth University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
395_2015_509_MOESM1_ESM.tif
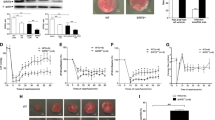
Supplementary material 1 Supplemental Fig.S1 At later reperfusion, MLS-STAT3E does not alter PAR polymers formation. Equal amounts of cytosolic extracts from ISC-REP and time control hearts from WT and MLS-STAT3E mice were resolved by SDS-PAGE, transferred to PVDF membrane and probed for PAR (Merck Millipore, Billerica, MA). GAPDH was used as protein loading control. Densitometry was measured using ImageJ (NIH, Bethesda) and expressed as ratio of probed protein signal to GAPDH signal and normalized to time control set as 1. Results are mean ± SEM, n = 6. *P < 0.05 vs. corresponding time control. The dotted bars reflect the data obtained under ISC-REP conditions. (TIFF 465 kb)
395_2015_509_MOESM2_ESM.tif
Supplementary material 2 Supplemental Fig.S2 STAT3 serine 727 phosphorylation status in the mitochondria from MLS-STAT3E and WT hearts subjected to 35 min ISC and 60 min REP. 30 μg of mitochondrial protein from WT hearts and 5 μg of mitochondrial protein from MLS-STAT3E hearts were separated by SDS-PAGE, transferred to PVDF membrane and probed for phosphorylated STAT3 serine 727 (p-Ser727-STAT3) and total STAT3. Porin was used as protein loading control. A representative immunoblot of three independent experiments is shown. (TIFF 1688 kb)
395_2015_509_MOESM3_ESM.tif
Supplementary material 3 Supplemental Fig.S3 MLS-STAT3E attenuates MPTP during early REP. MLS-STAT3E and WT hearts were subjected to ex vivo Langendorff model of 35 min ISC and 10 or 60 min REP followed by mitochondria isolation and assessment of Calcium Retention Capacity (CRC). CRC was evaluated in mitochondria by sequential pulses of calcium (5 nmol) in a presence of calcein green indicator. Results are mean ± SEM, n = 4. *P < 0.05 vs. 10 min REP, #P < 0.05 vs. WT. (TIFF 483 kb)
395_2015_509_MOESM4_ESM.tif
Supplementary material 4 Supplemental Fig.S4 MLS-STAT3E attenuates cytochrome c release into cytosol during early REP. Equal amounts of cytosolic extracts from WT and MLS-STAT3E hearts subjected to ex vivo Langendorff model of 35 min ISC and 10 REP were resolved by SDS-PAGE, transferred to PVDF membrane and probed for cytochrome c. GAPDH was used as protein loading control. Densitometry was measured using ImageJ (NIH, Bethesda) and expressed as ratio of probed protein signal to GAPDH signal. Results are mean ± SEM, n = 4. *P < 0.05 vs. WT. (TIFF 776 kb)
Rights and permissions
About this article
Cite this article
Szczepanek, K., Xu, A., Hu, Y. et al. Cardioprotective function of mitochondrial-targeted and transcriptionally inactive STAT3 against ischemia and reperfusion injury. Basic Res Cardiol 110, 53 (2015). https://doi.org/10.1007/s00395-015-0509-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-015-0509-2