Abstract
Purpose
Both folate and betaine (synthesized from choline) are nutrients used to methylate homocysteine to reform the amino acid methionine following donation of its methyl group; however, it is unclear whether both remethylation pathways are of equal importance during the neonatal period when remethylation rates are high. Methionine is an indispensable amino acid that is in high demand in neonates not only for protein synthesis, but is also particularly important for transmethylation reactions, such as creatine and phosphatidylcholine synthesis. The objective of this study was to determine whether supplementation with folate, betaine, or a combination of both can equally re-synthesize methionine for protein synthesis when dietary methionine is limiting.
Methods
Piglets were fed a low methionine diet devoid of folate, choline, and betaine, and on day 6, piglets were supplemented with either folate, betaine, or folate + betaine (n = 6 per treatment) until day 10. [1-13C]-phenylalanine oxidation was measured as an indicator of methionine availability for protein synthesis both before and after 2 days of supplementation.
Results
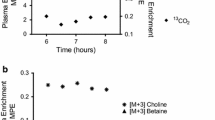
Prior to supplementation, piglets had lower concentrations of plasma folate, betaine, and choline compared to baseline with no change in homocysteine. Post-supplementation, phenylalanine oxidation levels were 20–46 % lower with any methyl donor supplementation (P = 0.006) with no difference among different supplementation groups. Furthermore, both methyl donors led to similarly lower concentrations of homocysteine following supplementation (P < 0.05).
Conclusions
These data demonstrate an equal capacity for betaine and folate to remethylate methionine for protein synthesis, as indicated by lower phenylalanine oxidation.


Similar content being viewed by others
References
Schubert HL, Blumenthal RM, Cheng X (2003) Many paths to methyl transfer: a chronicle of convergence. Trends Biochem Sci 28:329–335
Bauchart-Thevret C, Stoll B, Chackom S, Burrin DG (2009) Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am J Physiol Endocrinol Metab 296:1239–1250
Huang L, Hogewind-Schoonenboom JE, van Dongen MJ, de Groof F, Voortman GJ, Schierbeek H et al (2012) Methionine requirement of the enterally fed term infant in the first month of life in the presence of cysteine. Am J Clin Nutr 95:1048–1054
Shoveller AK, Brunton JA, House JD, Pencharz PB, Ball RO (2003) Dietary cysteine reduces the methionine requirement by an equal proportion in both parenterally and enterally fed piglets. J Nutr 133:4215–4224
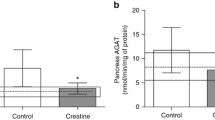
Brosnan JT, Wijekoon EP, Warford-Woolgar L, Trottier NL, Brosnan ME, Brunton JA, Bertolo RF (2009) Creatine synthesis is a major metabolic process in neonatal piglets and has important implications for amino acid metabolism and methyl balance. J Nutr 139:1292–1297
Plagemann A, Harder T, Brunn M, Harder A, Roepke K, Wittrock-Staar M et al (2009) Hypothalamicproopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol 587:4963–4976
Thomas B, Gruca LL, Bennett C, Parimi PS, Hanson RW, Kalhan SC (2008) Metabolism of methionine in the newborn infant: response to the parenteral and enteral administration of nutrients. Pediatr Res 64:381–386
Pajares MA, Pérez-Sala D (2006) Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism. CMLS 63:2792–2803
Finkelstein JD (1990) Methionine metabolism in mammals. J Nutr Biochem 1:228–237
Skiba WE, Taylor MP, Wells MS, Mangum JH, Awad WM Jr (1982) Human hepatic methionine biosynthesis. Purification and characterization of betaine:homocysteine S-methyltransferase. J Biol Chem 257:14944–14948
Wittwer AJ, Wagner C (1981) Identification of the folate-binding proteins of rat liver mitochondria as dimethylglycine dehydrogenase and sarcosine dehydrogenase. Flavoprotein nature and enzymatic properties of the purified proteins. J Biol Chem 256:4109–4115
Ilcol YO, Ozbek R, Hamurtekin E, Ulus IH (2005) Choline status in newborns, infants, children, breast-feeding women, breast-fed infants and human breast milk. J Nutr Biochem 16:489–499
Fischer LM, da Costa KA, Galanko J, Sha W, Stephenson B, Vick J, Zeisel SH (2010) Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am J Clin Nutr 92:336–346
Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Lewis DS et al (1994) Amino acid composition of human milk is not unique. J Nutr 124:1126–1132
Selhub J, Seyoum E, Pomfret EA, Zeisel SH (1991) Effects of choline deficiency and methotrexate treatment upon liver folate content and distribution. Cancer Res 51:16–21
Kim YI, Miller JW, da Costa KA, Nadeau M, Smith D, Selhub J et al (1994) Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J Nutr 124:2197–2203
Elango R, Ball RO, Pencharz PB (2008) Indicator amino acid oxidation: concept and application. J Nutr 138:243–246
Dodge ME, Bertolo RF, Brunton JA (2012) Enteral feeding induces early intestinal adaptation in a parenterally fed neonatal piglet model of short bowel syndrome. J Parenter Enter Nutr 36:205–212
Shoveller AK, Brunton JA, Pencharz PB, Ball RO (2003) The methionine requirement is lower in neonatal piglets fed parenterally than in those fed enterally. J Nutr 133:1390–1397
Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS et al (2004) Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab 286:E272–E279
Moehn S, Bertolo RF, Pencharz PB, Ball RO (2005) Development of the indicator amino acid oxidation technique to determine the availability of amino acids from dietary protein in pigs. J Nutr 135:2866–2870
Vester B, Rasmussen K (1991) High performance liquid chromatography method for rapid and accurate determination of homocysteine in plasma and serum. Eur J Clin Chem Clin Biochem 29:549–554
Bidlingmeyer BA, Cohen SA, Tarvin TL (1984) Rapid analysis of amino acids using pre-column derivatization. J Chromatogr 336:93–104
Holm PI, Ueland PM, Kvalheim G, Lien EA (2003) Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem 49:286–294
Kirsch SH, Herrmann W, Rabagny Y, Obeid R (2010) Quantification of acetylcholine, choline, betaine, and dimethylglycine in human plasma and urine using stable-isotope dilution ultra performance liquid chromatography–tandem mass spectrometry. J Chromatogr B 878:3338–3344
Lamarre SG, Saulnier RJ, Blier PU, Driedzic WR (2015) A rapid and convenient method for measuring the fractional rate of protein synthesis in ectothermic animal tissues using a stable isotope tracer. Comp Biochem Physiol B Biochem Mol Biol 182:1–5
Zello GA, Pencharz PB, Ball RO (1993) Dietary lysine requirement of young adult males determined by oxidation of l-[1-13C]phenylalanine. Am J Physiol 264:E677–E685
Wykes LJ, House JD, Ball RO, Pencharz PB (1994) Aromatic amino acid metabolism of neonatal piglets receiving TPN: effect of tyrosine precursors. Am J Physiol 267:E672–E679
House JD, Pencharz PB, Ball RO (1997) Phenylalanine requirements determined by using l-[1-14C]phenylalanine in neonatal piglets receiving total parenteral nutrition supplemented with tyrosine. Am J Clin Nutr 65:984–993
Finkelstein JD, Martin JJ (1984) Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J Biol Chem 259:9508–9513
Shinohara Y, Hasegawa H, Ogawa K, Tagoku K, Hashimoto T (2006) Distinct effects of folate and choline deficiency on plasma kinetics of methionine and homocysteine in rats. Metabolism 55:899–906
Asrar FM, O’Connor DL (2005) Bacterially synthesized folate and supplemental folic acid are absorbed across the large intestine of piglets. J Nutr Biochem 16:587–593
Kang SS, Wong PW, Norusis M (1987) Homocysteinemia due to folate deficiency. Metabolism 36:458–462
Miller J, Nadeau M, Smith J, Smith D, Selhub J (1994) Folate-deficiency-induced homocysteinaemia in rats: disruption of S-adenosylmethionine’s co-ordinate regulation of homocysteine metabolism. J Biochem 298:415–419
Teng Y, Cerdena I, Zeisel SH (2012) Homocysteinemia in mice with genetic betaine homocysteine S-methyltransferase deficiency is independent of dietary folate intake. J Nutr 142:1964–1967
Robinson JL, McBreairty LE, Harding SV, Randell EW, Brunton JA, Bertolo RF (2014) The dietary methyl donors folate, betaine and choline have a significant impact on the partitioning of methionine in the neonatal piglet. In: Canadian nutrition society annual conference, St. John’s, NL, June, 2014. Appl Physiol Nutr Metab 39(5):635 (Abstract). doi:10.1139/apnm-2014-0085
Refsum H, Grindflek AW, Ueland PM, Fredriksen A, Meyer K, Ulvik A et al (2004) Screening for serum total homocysteine in newborn children. Clin Chem 50:1769–1784
Acknowledgments
We thank M.E. Dodge for laboratory assistance. We would like to thank Dr. Simon Eaton at University College London’s Institute for Child Health for use of the isotope ratio mass spectrometer.
Financial support
This work was supported by the Canadian Institutes of Health Research (R.F.B., Grant Number 201103RNL); and the Research Development Corporation of Newfoundland and Labrador (R.F.B., Grant Number 5404-1046-104).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Authorship
L.E.M., J.L.R., R.F.B., and J.A.B. were involved in formulating the research question and designing the experiment. L.E.M. and J.L.R. carried out the experiments, and L.E.M., J.L.R., S.V.H., and E.W.R. conducted the analytical work. L.E.M., J.L.R., R.F.B., and J.A.B. analyzed and interpreted the data. L.E.M. and R.F.B. drafted the manuscript, and all authors read and reviewed the final version.
Rights and permissions
About this article
Cite this article
McBreairty, L.E., Robinson, J.L., Harding, S.V. et al. Betaine is as effective as folate at re-synthesizing methionine for protein synthesis during moderate methionine deficiency in piglets. Eur J Nutr 55, 2423–2430 (2016). https://doi.org/10.1007/s00394-015-1049-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1049-0