Abstract
Purpose
The aim was to determine the effects of cafeteria diet (CD) and fish oil supplements given to pregnant and lactating rats on the birth weight and fatty acid profiles of their offspring.
Methods
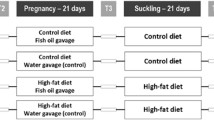
Female rats were given standard diet (STD) or CD for 22 days before pregnancy. After mating, some animals remained on STD or CD; for some CD rats, the diet was supplemented with 8.78 % fish oil (CD-FO). After 12 days, half the CD-FO group returned to CD (CD-FO12) and the others remained on CD-FO.
Results
At birth, body weights of pups of the three CD groups were lower than STD, maintained until 21 days in the CD-FO group only. At the end of lactation, dams of the CD groups had increased plasma triacylglycerols (TAG), non-esterified fatty acids, and glycerol concentrations, whereas most n-6 long-chain polyunsaturated fatty acids (LCPUFA) were decreased, the effect being greatest in the CD-FO group, where most n-3 LCPUFA were increased and indices of Δ5 and Δ6 desaturase activities decreased. The 21-day-old pups of the CD group had increased plasma TAG, not present in the CD-FO group, which had increased 3-hydroxybutyrate concentrations. In both 2- and 21-day-old CD pups, plasma concentrations of ARA were lower than STD, and even lower in the two CD-FO groups.
Conclusions
The effect of CD and CD-FO decreasing pups body weight could be related to decreased concentrations of ARA, caused by the inhibition of the Δ5 and Δ6 desaturases in the pathway of n-6 LCPUFA biosynthesis.

Similar content being viewed by others
References
Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, Saade G, Eddleman K, Carter SM, Craigo SD, Carr SR, D’Alton ME, Consortium FR (2004) Obesity, obstetric complications and cesarean delivery rate-a population-based screening study. Am J Obstet Gynecol 190:1091–1097
Walsh SW (2007) Obesity: a risk factor for preeclampsia. Trends Endocrinol Metab 18:365–370
Dixit A, Girling JC (2008) Obesity and pregnancy. J Obstet Gynaecol 28:14–23
Chu SY, Kim SY, Schmid CH, Dietz PM, Callaghan WM, Lau J, Curtis KM (2007) Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev 8:385–394
Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S (2001) Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 25:1175–1182
Jansson N, Nilsfelt A, Gellerstedt M, Wennergren M, Rossander-Hulthen L, Powell TL, Jansson T (2008) Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr 87:1743–1749
Cnattingius S, Bergstrom R, Lipworth L, Kramer MS (1998) Pre-pregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med 338:147–152
Perlow JH, Morgan MA, Montgomery D, Towers CV, Porto M (1992) Perinatal outcome in pregnancy complicated by massive obesity. Am J Obstet Gynecol 167:958–962
McDonald SD, Han Z, Mulla S, Beyene J, Knowledge Synthesis Group (2010) Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. Br Med J 341:c3428. doi:10.1136/bmj.c3428
Armitage JA, Taylor PD, Poston L (2005) Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol 565:3–8
Holemans K, Caluwaerts S, Poston L, Van Assche FA (2004) Diet-induced obesity in the rat: a model for gestational diabetes mellitus. Am J Obstet Gynecol 190:858–865
Ozanne SE, Lewis R, Jennings BJ, Hales CN (2004) Early programming of weight gain in mice prevents the induction of obesity by a highly palatable diet. Clin Sci 106:141–145
Akyol A, McMullen S, Langley-Evans SC (2012) Glucose intolerance associated with early-life exposure to maternal cafeteria feeding is dependent upon post-weaning diet. Br J Nutr 107:964–978
Akyol A, Langley-Evans SC, McMullen S (2009) Obesity induced by cafeteria feeding and pregnancy outcome in the rat. Br J Nutr 102:1601–1610
Bayol SA, Simbi BH, Stickland NC (2005) A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol 567:951–961
Neuringer M, Connor WE, Van Petten C, Barstad L (1984) Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J Clin Invest 73:272–276
Gibson RA, Makrides M (1999) Polyunsaturated fatty acids and infant visual development: a critical appraisal of randomized clinical trials. Lipids 34:179–184
Carlson SE, Neuringer M (1999) Polyunsaturated fatty acid status and neurodevelopment: a summary and critical analysis of the literature. Lipids 34:171–178
Hodge L, Salome CM, Peat JK, Haby MM, Xuan W, Woolcock AJ (1996) Consumption of oily fish and childhood asthma risk. Med J Aust 164:137–140
Black PN, Sharpe S (1997) Dietary fat and asthma: is there a connection? Eur Respir J 10:6–12
van Houwelingen AC, Sorensen JD, Hornstra G, Simonis MM, Boris J, Olsen SF, Secher NJ (1995) Essential fatty acid status in neonates after fish-oil supplementation during late pregnancy. Br J Nutr 74:723–731
Helland IB, Saugstad OD, Smith L, Saarem K, Solvoll K, Ganes T, Drevon CA (2001) Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics 108:E82
Velzing-Aarts FV, van der Klis FR, van der Dijs FP, van Beusekom CM, Landman H, Capello JJ, Muskiet FA (2001) Effect of three low-dose fish oil supplements, administered during pregnancy, on neonatal long-chain polyunsaturated fatty acid status at birth. Prostaglandins Leukot Essent Fatty Acids 65:51–57
Escolano-Margarit MV, Campoy C, Ramirez-Tortosa MC, Demmelmair H, Miranda MT, Gil A, Decsi T, Koletzko BV (2013) Effects of fish oil supplementation on the fatty acid profile in erythrocyte membrane and plasma phospholipids of pregnant women and their offspring: a randomised controlled trial. Br J Nutr 109:1647–1656
Ribeiro P, Carvalho FD, Abreu Ade A, Sant’anna Mde T, Lima RJ, Carvalho Pde O (2012) Effect of fish oil supplementation in pregnancy on the fatty acid composition of erythrocyte phospholipids and breast milk lipids. Int J Food Sc Nutr 63:36–40
Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, Makrides M (2012) Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in first year of life: randomised controlled trial. BMJ 344:e184
Beblo S, Reinhardt H, Demmelmair H, Muntau AC, Koletzko B (2007) Effect of fish oil supplementation on fatty acid status, coordination, and fine motor skills in children with phenylketonuria. J Pediatr 150:479–484
Metcalf RG, James MJ, Gibson RA, Edwards JR, Stubberfield J, Stuklis R, Roberts-Thomson K, Young GD, Cleland LG (2007) Effects of fish-oil supplementation on myocardial fatty acids in humans. Am J Clin Nutr 85:1222–1228
Dunstan JA, Mori TA, Barden A, Beilin LJ, Holt PG, Calder PC, Taylor AL, Prescott SL (2004) Effects of n-3 polyunsaturated fatty acid supplementation in pregnancy on maternal and fetal erythrocyte fatty acid composition. Eur J Clin Nutr 58:429–437
Amusquivar E, Ruperez FJ, Barbas C, Herrera E (2000) Low arachidonic acid rather than alpha-tocopherol is responsible for the delayed postnatal development in offspring of rats fed fish oil instead of olive oil during pregnancy and lactation. J Nutr 130:2855–2865
Koletzko B, Braun M (1991) Arachidonic acid and early human growth: is there a relation? Ann Nutr Metab 35:128–131
Clandinin MT, VanAerde J (2003) Formula supplementation and growth. Pediatrics 112:1456–1458
Bouanane S, Merzouk H, Benkalfat NB, Soulimane N, Merzouk SA, Gresti J, Tessier C, Narce M (2010) Hepatic and very low-density lipoprotein fatty acids in obese offspring of overfed dams. Metabolism 59:1701–1709
Sampson DA, Jansen GR (1984) Measurement of milk yield in the lactating rat from pup weight and weight gain. J Pediatr Gastroenterol Nutr 3:613–617
Williamson DH, Mellanby J, Krebs HA (1962) Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J 82:90–96
International OMOAOA (2007) AOAC International Gaithersburg, MD, USA
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Amusquivar E, Schiffner S, Herrera E (2011) Evaluation of two methods for plasma fatty acid analysis by GC. Eur J Lipid Sci Technol 113:711–716
Leddy MA, Power ML, Schulkin J (2008) The impact of maternal obesity on maternal and fetal health. Rev Obst Gynecol 1:170–178
Whitaker RC (2004) Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 114:e29–e36
Niculescu MD, Lupu DS (2009) High fat diet-induced maternal obesity alters fetal hippocampal development. Int J Dev Neurosci 27:627–633
Mendes-da-Silva C, Giriko CA, Mennitti LV, Hosoume LF, Souto Tdos S, da Silva AV (2014) Maternal high-fat diet during pregnancy or lactation changes the somatic and neurological development of the offspring. Arq Neuropsiquiatr 72:136–144
Bayol SA, Simbi BH, Fowkes RC, Stickland NC (2010) A maternal “junk food” diet in pregnancy and lactation promotes nonalcoholic Fatty liver disease in rat offspring. Endocrinology 151:1451–1461
Langley-Evans SC, Nwagwu M (1998) Impaired growth and increased glucocorticoid-sensitive enzyme activities in tissues of rat fetuses exposed to maternal low protein diets. Life Sci 63:605–615
Bieswal F, Ahn MT, Reusens B, Holvoet P, Raes M, Rees WD, Remacle C (2006) The importance of catch-up growth after early malnutrition for the programming of obesity in male rat. Obesity 14:1330–1343
IfLA Research (1995) Nutrient requirements of laboratory animals. National Academies Press, Washington, DC
Hornstra G (2000) Essential fatty acids in mothers and their neonates. Am J Clin Nutr 71:1262S–1269S
Koletzko B, Agostoni C, Carlson SE, Clandinin T, Hornstra G, Neuringer M, Uauy R, Yamashiro Y, Willatts P (2001) Long chain polyunsaturated fatty acids (LC-PUFA) and perinatal development. Acta Paediatr 90:460–464
Crawford MA (2000) Placental delivery of arachidonic and docosahexaenoic adis: implications for the lipid nutrition of preterm infants. Am J Clin Nutr 7(Suppl 1):275S–284S
Hostmark AT, Haug A (2013) Percentage oleic acid is inversely related to percentage arachidonic acid in total lipids of rat serum. Lipids Health Dis 12:40. doi:10.1186/1476-511X-12-40
Saste MD, Carver JD, Stockard JE, Benford VJ, Chen LT, Phelps CP (1998) Maternal diet fatty acid composition affects neurodevelopment in rat pups. J Nutr 128:740–743
Christiansen EN, Lund JS, Rortveit T, Rustan AC (1991) Effect of dietary n-3 and n-6 fatty acids on fatty acid desaturation in rat liver. Biochim Biophys Acta 1082:57–62
Raz A, Kamin-Belsky N, Przedecki F, Obukowicz MG (1997) Fish oil inhibits delta 6 desaturase activity in vivo. Utility in a dietary paradigm to obtain mice depleted of arachidonic acid. J Nutr Biochem 8:558–565
Herrera E, Ortega-Senovilla H (2014) Lipid metabolism during pregnancy and its implications for fetal growth. Curr Pharm Biotechnol 15:24–31
Amusquivar E, Laws J, Clarke L, Herrera E (2010) Fatty acid composition of the maternal diet during the first or the second half of gestation influences the fatty acid composition of sows’ milk and plasma, and plasma of their piglets. Lipids 45:409–418
Fernandes FS, do Carmo MT, Herrera E (2012) Influence of maternal diet during early pregnancy on the fatty acid profile in the fetus at late pregnancy in rats. Lipids 47:505–517
Lalanza JF, Caimari A, del Bas JM, Torregrosa D, Cigarroa I, Pallas M, Capdevila L, Arola L, Escorihuela RM (2014) Effects of a post-weaning cafeteria diet in young rats: metabolic syndrome, reduced activity and low anxiety-like behaviour. PLoS ONE 9:e85049. doi:10.1371/journal.pone.0085049
Mucellini AB, Goularte JF, de Araujo da Cunha AC, Caceres RC, Noschang C, da Silva Benetti C, Silveira PP, Sanvitto GL (2014) Effects of exposure to a cafeteria diet during gestation and after weaning on the metabolism and body weight of adult male offspring in rats. Br J Nutr 111:1499–1506
Vanzela EC, Ribeiro RA, de Oliveira CA, Rodrigues FB, Bonfleur ML, Carneiro EM, Souza KL, Boschero AC (2010) Pregnancy restores insulin secretion from pancreatic islets in cafeteria diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol 298:R320–R328
Innis SM, Rioux FM, Auestad N, Ackman RG (1995) Marine and freshwater fish oil varying in arachidonic, eicosapentaenoic and docosahexaenoic acids differ in their effects on organ lipids and fatty acids in growing rats. J Nutr 125:2286–2293
Wong SH, Nestel PJ, Trimble RP, Storer GB, Illman RJ, Topping DL (1984) The adaptive effects of dietary fish and safflower oil on lipid and lipoprotein metabolism in perfused rat liver. Biochim Biophys Acta 792:103–109
Rasmussen KM (1998) Effects of under- and overnutrition on lactation in laboratory rats. J Nutr 128:390S–393S
Del Prado M, Delgado G, Villalpando S (1997) Maternal lipid intake during pregnancy and lactation alters milk composition and production and litter growth in rats. J Nutr 127:458–462
Bazinet RP, Laye S (2014) Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci 15:771–785
Acknowledgments
The authors thank Milagros Morante for excellent technical help, Dr. Purificación González González for her valuable help in the analysis of the composition of the diets, and pp-science-editing.com for editing and linguistic revision of the manuscript. The study has been carried out with the financial support of Fundación Ramón Areces (CIVP16A1835) and University CEU San Pablo (PPC03-2014), Madrid, Spain. This manuscript does not contain clinical studies or patient data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors had any financial or personal conflicts of interest.
Rights and permissions
About this article
Cite this article
Sánchez-Blanco, C., Amusquivar, E., Bispo, K. et al. Influence of cafeteria diet and fish oil in pregnancy and lactation on pups’ body weight and fatty acid profiles in rats. Eur J Nutr 55, 1741–1753 (2016). https://doi.org/10.1007/s00394-015-0992-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0992-0