Abstract
Purpose
Exercise induces oxidative stress and causes adaptations in antioxidant defenses. The aim of the present study was to determine the effects of a 2-month diet supplementation with docosahexaenoic acid (DHA) on the pro-oxidant and antioxidant status of peripheral blood mononuclear cells (PBMCs) during football training and after acute exercise.
Methods
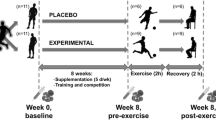
Fifteen male football players, in a randomized double-blind trial, ingested a beverage enriched with DHA or a placebo for 8 weeks. Blood samples were collected in basal conditions before and after the training period and after an acute and intense exercise.
Results
The training season increased the carbonyl and nitrotyrosine index but decreased the malondialdehyde (MDA) levels. Basal catalase activity decreased in both groups after 8 weeks of training, whereas glutathione peroxidase activity increased mainly in the placebo group. Protein levels of uncoupling proteins (UCP2 and UCP3) and inducible nitric oxide synthase significantly increased after the training period. Acute exercise induced redistribution in the number of circulating cells, increased the MDA levels and nitrotyrosine index, and decreased the levels of nitrate. Acute exercise also increased PBMCs reactive oxygen species (ROS) production after immune stimulation. Diet supplementation with DHA significantly increased the UCP3 levels after training and the superoxide dismutase protein levels after acute exercise, and reduced the production of ROS after acute exercise.
Conclusion
Docosahexaenoic acid increased the antioxidant capabilities while reducing the mitochondrial ROS production in a regular football training period and reduced the oxidative damage markers in response to acute exercise.


Similar content being viewed by others
References
Costill DL, Wilmore JH (2004) Fisiología del esfuerzo y del deporte. Paidotribo, Barcelona
Ji LL (1995) Oxidative stress during exercise: implication of antioxidant nutrients. Free Radic Biol Med 18:1079–1086
Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M (2013) Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal 18:1208–1246. doi:10.1089/ars.2011.4498
Viña J, Gimeno A, Sastre J, Desco C, Asensi M, Pallardó FV, Cuesta A, Ferrero JA, Terada LS, Repine JE (2000) Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life 49:539–544. doi:10.1080/15216540050167098
Sureda A, Batle JM, Ferrer MD, Mestre-Alfaro A, Tur JA, Pons A (2012) Scuba diving activates vascular antioxidant system. Int J Sports Med 33:531–536. doi:10.1055/s-0031-1297957
Wagner KH, Reichhold S, Neubauer O (2011) Impact of endurance and ultraendurance exercise on DNA damage. Ann N Y Acad Sci 1229:115–123. doi:10.1111/j.1749-6632.2011.06106.x
Nikolaidis MG, Jamurtas AZ (2009) Blood as a reactive species generator and redox status regulator during exercise. Arch Biochem Biophys 490:77–84. doi:10.1016/j.abb.2009.08.015
Ferrer MD, Sureda A, Mestre A, Tur JA, Pons A (2010) The double edge of reactive oxygen species as damaging and signaling molecules in HL60 cell culture. Cell Physiol Biochem 25:241–252. doi:10.1159/000276558
Alessio HM, Goldfarb AH (1988) Lipid peroxidation and scavenger enzymes during exercise: adaptive response to training. J Appl Physiol 64:1333–1336
Korzeniowska-Kubacka I, Bilińska M, Piotrowicz R (2007) Influence of physical training on cardiac performance in patients with coronary artery disease and exercise-induced left ventricular dysfunction. Acta Cardiol 62:573–578
Nicks CR, Morgan DW, Fuller DK, Caputo JL (2009) The influence of respiratory muscle training upon intermittent exercise performance. Int J Sports Med 30:16–21. doi:10.1055/s-2008-1038794
Tian Y, Nie J, Tong TK, Baker JS, Thomas NE, Shi Q (2010) Serum oxidant and antioxidant status during early and late recovery periods following an all-out 21-km run in trained adolescent runners. Eur J Appl Physiol 110:971–976. doi:10.1007/s00421-010-1583-7
Cases N, Aguiló A, Tauler P, Sureda A, Llompart I, Pons A, Tur JA (2005) Differential response of plasma and immune cell’s vitamin E levels to physical activity and antioxidant vitamin supplementation. Eur J Clin Nutr 59:781–788. doi:10.1038/sj.ejcn.1602143
Vina J, Borras C, Gomez-Cabrera MC, Orr WC (2006) Part of the series: from dietary antioxidants to regulators in cellular signalling and gene expression. Role of reactive oxygen species and (phyto)oestrogens in the modulation of adaptive response to stress. Free Radic Res 40:111–119. doi:10.1080/10715760500405778
Sureda A, Tauler P, Aguiló A, Fuentespina E, Córdova A, Tur JA, Pons A (2006) Blood cell NO synthesis in response to exercise. Nitric Oxide 15:5–12. doi:10.1016/j.niox.2005.11.004
Tauler P, Sureda A, Cases N, Aguiló A, Rodríguez-Marroyo JA, Villa G, Tur JA, Pons A (2006) Increased lymphocyte antioxidant defences in response to exhaustive exercise do not prevent oxidative damage. J Nutr Biochem 17:665–671. doi:10.1016/j.jnutbio.2005.10.013
Ferrer MD, Sureda A, Batle JM, Tauler P, Tur JA, Pons A (2007) Scuba diving enhances endogenous antioxidant defenses in lymphocytes and neutrophils. Free Radic Res 41:274–281. doi:10.1080/10715760601080371
Cortright RN, Zheng D, Jones JP, Fluckey JD, DiCarlo SE, Grujic D, Lowell BB, Dohm GL (1999) Regulation of skeletal muscle UCP-2 and UCP-3 gene expression by exercise and denervation. Am J Physiol 276:E217–E221
Sureda A, Tauler P, Aguiló A, Cases N, Llompart I, Tur JA, Pons A (2008) Influence of an antioxidant vitamin-enriched drink on pre- and post-exercise lymphocyte antioxidant system. Ann Nutr Metab 52:233–240. doi:10.1159/000140515
Uauy-Dagach R, Mena P, Hoffman DR (1994) Essential fatty acid metabolism and requirements for LBW infants. Acta Paediatr Suppl 405:78–85
Maki KC, Van Elswyk ME, McCarthy D, Hess SP, Veith PE, Bell M, Subbaiah P, Davidson MH (2005) Lipid responses to a dietary docosahexaenoic acid supplement in men and women with below average levels of high density lipoprotein cholesterol. J Am Coll Nutr 24:189–199
Crawford MA (2006) Docosahexaenoic acid in neural signaling systems. Nutr Health 18:263–276
Innis SM (2007) Dietary (n-3) fatty acids and brain development. J Nutr 137:855–859
Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Investigators JElisJ (2007) Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369:1090–1098
Mori TA, Codde JP, Vandongen R, Beilin LJ (1987) New findings in the fatty acid composition of individual platelet phospholipids in man after dietary fish oil supplementation. Lipids 22:744–750
Dawczynski C, Hackermeier U, Viehweger M, Stange R, Springer M, Jahreis G (2011) Incorporation of n-3 PUFA and γ-linolenic acid in blood lipids and red blood cell lipids together with their influence on disease activity in patients with chronic inflammatory arthritis–a randomized controlled human intervention trial. Lipids Health Dis 10:130. doi:10.1186/1476-511X-10-130
Tur JA, Bibiloni MM, Sureda A, Pons A (2012) Dietary sources of omega 3 fatty acids: public health risks and benefits. Br J Nutr 107(Suppl 2):S23–S52. doi:10.1017/S0007114512001456
Tartibian B, Maleki BH, Abbasi A (2011) Omega-3 fatty acids supplementation attenuates inflammatory markers after eccentric exercise in untrained men. Clin J Sport Med 21:131–137. doi:10.1097/JSM.0b013e31820f8c2f
Lorente-Cebrián S, Bustos M, Marti A, Martinez JA, Moreno-Aliaga MJ (2009) Eicosapentaenoic acid stimulates AMP-activated protein kinase and increases visfatin secretion in cultured murine adipocytes. Clin Sci 117:243–249. doi:10.1042/CS20090020
Motawi TM, Hashem RM, Rashed LA, El-Razek SM (2009) Comparative study between the effect of the peroxisome proliferator activated receptor-alpha ligands fenofibrate and n-3 polyunsaturated fatty acids on activation of 5′-AMP-activated protein kinase-alpha1 in high-fat fed rats. J Pharm Pharmacol 61:1339–1346. doi:10.1211/jpp/61.10.0010
Siddiqui RA, Harvey K, Stillwell W (2008) Anticancer properties of oxidation products of docosahexaenoic acid. Chem Phys Lipids 153:47–56. doi:10.1016/j.chemphyslip.2008.02.009
Filaire E, Massart A, Portier H, Rouveix M, Rosado F, Bage AS, Gobert M, Durand D (2010) Effect of 6 Weeks of n-3 fatty-acid supplementation on oxidative stress in Judo athletes. Int J Sport Nutr Exerc Metab 20:496–506
Richard D, Kefi K, Barbe U, Bausero P, Visioli F (2008) Polyunsaturated fatty acids as antioxidants. Pharmacol Res 57:451–455. doi:10.1016/j.phrs.2008.05.002
Di Nunzio M, Valli V, Bordoni A (2011) Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot Essent Fatty Acids 85:121–127. doi:10.1016/j.plefa.2011.07.005
Tholstrup T, Hellgren LI, Petersen M, Basu S, Straarup EM, Schnohr P, Sandström B (2004) A solid dietary fat containing fish oil redistributes lipoprotein subclasses without increasing oxidative stress in men. J Nutr 134:1051–1057
Sureda A, Ferrer MD, Tauler P, Romaguera D, Drobnic F, Pujol P, Tur JA, Pons A (2009) Effects of exercise intensity on lymphocyte H2O2 production and antioxidant defences in soccer players. Br J Sports Med 43:186–190
Mack GW, Yang R, Hargens AR, Nagashima K, Haskell A (1998) Influence of hydrostatic pressure gradients on regulation of plasma volume after exercise. J Appl Physiol 85:667–675
Tauler P, Ferrer MD, Romaguera D, Sureda A, Aguilo A, Tur J, Pons A (2008) Antioxidant response and oxidative damage induced by a swimming session: influence of gender. J Sports Sci 26:1303–1311. doi:10.1080/02640410801974992
Boyum A (1964) Separation of white blood cells. Nature 204:793–794
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Braman RS, Hendrix SA (1989) Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium (III) reduction with chemiluminescence detection. Anal Chem 61:2715–2718
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Goldberg DM Spooner R (1984) Glutathione reductase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Enzymes 1: oxidoreductases, transferases, vol 3. Verlag chemie, Basel, pp 258–265
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Toft AD, Thorn M, Ostrowski K, Asp S, Moller K, Iversen S, Hermann C, Sondergaard SR, Pedersen BK (2000) N-3 polyunsaturated fatty acids do not affect cytokine response to strenuous exercise. J Appl Physiol 89:2401–2406
Ferrer MD, Tauler P, Sureda A, Pujol P, Drobnic F, Tur JA, Pons A (2009) A soccer match’s ability to enhance lymphocyte capability to produce ROS and induce oxidative damage. Int J Sport Nutr Exerc Metab 19:243–258
Blough N, Zafkiou O (1985) Reaction of superoxide with nitric oxide to form peroxonitrite in alkaline aqueous solution. Inorg Chem 24:3502–3504
Radi R, Beckman JS, Bush KM, Freeman BA (1991) Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288:481–487
Radi R, Beckman JS, Bush KM, Freeman BA (1991) Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem 266:4244–4250
Moreno JJ, Pryor WA (1992) Inactivation of alpha 1-proteinase inhibitor by peroxynitrite. Chem Res Toxicol 5:425–431
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624
Kastle M, Grune T (2011) Protein oxidative modification in the aging organism and the role of the ubiquitin proteasomal system. Curr Pharm Des 17:4007–4022
Miyata T, Inagi R, Asahi K, Yamada Y, Horie K, Sakai H, Uchida K, Kurokawa K (1998) Generation of protein carbonyls by glycoxidation and lipoxidation reactions with autoxidation products of ascorbic acid and polyunsaturated fatty acids. FEBS Lett 437:24–28
Ferrer MD, Tauler P, Sureda A, Tur JA, Pons A (2009) Antioxidant regulatory mechanisms in neutrophils and lymphocytes after intense exercise. J Sports Sci 27:49–58. doi:10.1080/02640410802409683
Siess H (1985) Oxidative stress. Academic Press, London
Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD (2002) Superoxide activates mitochondrial uncoupling proteins. Nature 415:96–99. doi:10.1038/415096a
Cannon B, Shabalina IG, Kramarova TV, Petrovic N, Nedergaard J (2006) Uncoupling proteins: a role in protection against reactive oxygen species—or not? Biochim Biophys Acta 1757:449–458. doi:10.1016/j.bbabio.2006.05.016
Jiang N, Zhang G, Bo H, Qu J, Ma G, Cao D, Wen L, Liu S, Ji LL, Zhang Y (2009) Upregulation of uncoupling protein-3 in skeletal muscle during exercise: a potential antioxidant function. Free Radic Biol Med 46:138–145. doi:10.1016/j.freeradbiomed.2008.09.026
Hamanaka RB, Chandel NS (2010) Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 35:505–513. doi:10.1016/j.tibs.2010.04.002
Tauler P, Aguiló A, Gimeno I, Noguera A, Agustí A, Tur JA, Pons A (2003) Differential response of lymphocytes and neutrophils to high intensity physical activity and to vitamin C diet supplementation. Free Radic Res 37:931–938
Okutsu M, Suzuki K, Ishijima T, Peake J, Higuchi M (2008) The effects of acute exercise-induced cortisol on CCR2 expression on human monocytes. Brain Behav Immun 22:1066–1071. doi:10.1016/j.bbi.2008.03.006
Zararsiz I, Sonmez MF, Yilmaz HR, Tas U, Kus I, Kavakli A, Sarsilmaz M (2006) Effects of omega-3 essential fatty acids against formaldehyde-induced nephropathy in rats. Toxicol Ind Health 22:223–229
Kesavulu MM, Kameswararao B, Apparao C, Kumar EG, Harinarayan CV (2002) Effect of omega-3 fatty acids on lipid peroxidation and antioxidant enzyme status in type 2 diabetic patients. Diabetes Metab 28:20–26
Sureda A, Tauler P, Aguiló A, Cases N, Fuentespina E, Córdova A, Tur JA, Pons A (2005) Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res 39:1317–1324. doi:10.1080/10715760500177500
Sureda A, Ferrer MD, Mestre A, Tur JA, Pons A (2013) Prevention of neutrophil protein oxidation with vitamins C and e diet supplementation without affecting the adaptive response to exercise. Int J Sport Nutr Exerc Metab 23:31–39
Bescós R, Rodríguez FA, Iglesias X, Ferrer MD, Iborra E, Pons A (2011) Acute administration of inorganic nitrate reduces VO(2peak) in endurance athletes. Med Sci Sports Exerc 43:1979–1986. doi:10.1249/MSS.0b013e318217d439
Tauler P, Aguiló A, Guix P, Jiménez F, Villa G, Tur JA, Córdova A, Pons A (2005) Pre-exercise antioxidant enzyme activities determine the antioxidant enzyme erythrocyte response to exercise. J Sports Sci 23:5–13. doi:10.1080/02640410410001716724
Mestre-Alfaro A, Ferrer MD, Sureda A, Tauler P, Martínez E, Bibiloni MM, Micol V, Tur JA, Pons A (2011) Phytoestrogens enhance antioxidant enzymes after swimming exercise and modulate sex hormone plasma levels in female swimmers. Eur J Appl Physiol 111:2281–2294. doi:10.1007/s00421-011-1862-y
Carrera-Quintanar L, Funes L, Viudes E, Tur J, Micol V, Roche E, Pons A (2012) Antioxidant effect of lemon verbena extracts in lymphocytes of university students performing aerobic training program. Scand J Med Sci Sports 22:454–461. doi:10.1111/j.1600-0838.2010.01244.x
Wang P, Wu Y, Li X, Ma X, Zhong L (2013) Thioredoxin and thioredoxin reductase control tissue factor activity by thiol redox-dependent mechanism. J Biol Chem 288:3346–3358. doi:10.1074/jbc.M112.418046
Benhar M, Forrester MT, Hess DT, Stamler JS (2008) Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science 320:1050–1054. doi:10.1126/science.1158265
Sureda A, Ferrer MD, Tauler P, Tur JA, Pons A (2008) Lymphocyte antioxidant response and H2O2 production after a swimming session: gender differences. Free Radic Res 42:312–319. doi:10.1080/10715760801989926
Phillips MD, Flynn MG, McFarlin BK, Stewart LK, Timmerman KL, Ji H (2008) Resistive exercise blunts LPS-stimulated TNF-alpha and Il-1 beta. Int J Sports Med 29:102–109. doi:10.1055/s-2007-965115
Starkie RL, Rolland J, Angus DJ, Anderson MJ, Febbraio MA (2001) Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-alpha levels after prolonged running. Am J Physiol Cell Physiol 280:C769–C774
Cha SH, Fukushima A, Sakuma K, Kagawa Y (2001) Chronic docosahexaenoic acid intake enhances expression of the gene for uncoupling protein 3 and affects pleiotropic mRNA levels in skeletal muscle of aged C57BL/6NJcl mice. J Nutr 131:2636–2642
Müller JM, Ziegler-Heitbrock HW, Baeuerle PA (1993) Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology 187:233–256. doi:10.1016/S0171-2985(11)80342-6
Emre Y, Hurtaud C, Nübel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM (2007) Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J 402:271–278. doi:10.1042/BJ20061430
Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E (2004) Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol 172:2522–2529
Baillie RA, Takada R, Nakamura M, Clarke SD (1999) Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostaglandins Leukot Essent Fatty Acids 60:351–356
Acknowledgments
Acción Estratégica en Salud del Ministro de Ciencia e Innovación DPS2008-07033-C03-03, Programme of Promotion of Biomedical Research and Health Sciences, Projects 11/01791, Red Predimed-RETIC RD06/0045/1004, CIBERobn CB12/03/30038 and Balearic Island Government and FEDER funds (35/2011 and 23/2012). We would like to thank the football players involved in the study for their committed participation. The excellent technical and practical assistance of Tomeu Munar, Cédric Thyus and RCD Mallorca is appreciated.
Conflict of interest
The authors declare that they do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Capó, X., Martorell, M., Sureda, A. et al. Diet supplementation with DHA-enriched food in football players during training season enhances the mitochondrial antioxidant capabilities in blood mononuclear cells. Eur J Nutr 54, 35–49 (2015). https://doi.org/10.1007/s00394-014-0683-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0683-2