Abstract
Background
Implantable cardioverter defibrillator (ICD) is a life-saving therapy for patients at risk of ventricular arrhythmias. Due to its high cost, its cost-effectiveness is highly dependent on its longevity, which is currently only based upon the manufacturer’s predicted device life span.
Aim
We sought to assess the ICDs’ longevity and the factors influencing it, and to compare the observed (real life) to the expected (manufacturer’s prediction) life span at a device level.
Methods
We retrospectively identified all patients who underwent an ICD implantation in a tertiary care medical center. For each device, an expected longevity was assigned based on the manufacturer/model, pacing percentage, and number of shocks per year. We defined device failure if the observed survival was shorter than 80 % of the expected. Only devices with follow-up time that exceeded the expected longevity were included.
Results
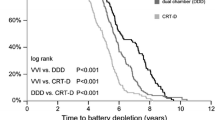
Of the 275 devices in the cohort, 79 (29 %) failed. Median device longevity was 5 years and varied markedly between manufacturers (4.3, 4.8, 5.1, and 6.3 years for Biotronik, St. Jude Medical, Boston Scientific, and Medtronic, respectively). There were significant differences among the manufacturers in device failure rates: 48, 17, 22, and 14 % for Biotronik, St. Jude Medical, Boston Scientific, and Medtronic, respectively). In multivariate analysis, manufacturer, earlier year of implantation, congestive heart failure and chronic renal failure significantly predicted device failure.
Conclusions
In conclusion, there is a significant device failure rate among ICDs, with variability among manufacturers, impacting both patients and the medical economic systems.




Similar content being viewed by others
Abbreviations
- ICD:
-
Implantable cardioverter defibrillator
- VVI:
-
Single chamber ICD
- DDD:
-
Dual chamber ICD
- CRT:
-
Cardiac resynchronization therapy bi-ventricular ICD
- ERI:
-
Elective replacement indication
- QALY:
-
Quality-adjusted life-years
References
Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins ST, Klein H et al (1996) Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med 335:1933–1940
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber D, Cannom DS et al (2002) Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346:877–883
Kadish A, Dyer A, Daubert JP, Quigg R, Estes M, Anderson KP et al (2004) Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 350:2151–2158
Epstein AE, DiMarco JP, Ellenbogen KA, Estes M, Freedman RA, Gettes LS et al (2008) ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm 5:e1–e62
Sanders GD, Hlatky MA, Owens DK (2005) Cost-effectiveness of implantable cardioverter–defibrillators. N Engl J Med 353:1471–1480
Hauser RG (2005) The growing mismatch between patient longevity and the service life of implantable cardioverter-defibrillators. J Am Coll Cardiol 45:2022–2025
Biffi M, Ziacchi M, Bertini M, Sangoirgi D, Corsini D, Martignan C et al (2008) Longevity of implantable cardioverter-defibrillators: implications for clinical practice and health care systems. Europace 10:1288–1295
Ellinor PT, Guy ML, Ruskin JN, McGovern BA (2003) Variability in implantable cardioverter defibrillator pulse generator longevity between manufacturers. Pacing Clin Electrophysiol 26:71–75
Gepner K, Przybylski A, Maciag A, Sterliński M, Lewandowski M, Syska P et al (2007) Causes of redo procedures in patients with an implantable cardioverter-defibrillator—long-term follow-up results. Kardiol Pol 65:893–898
Schaer BA, Koller MT, Sticherling C, Altmann D, Joerg L, Osswald S (2009) Longevity of implantable cardiovascular-defibrillators, influencing factors, and comparison to industry-projected longevity. Heart Rhythm 6:1737–1743
Medtronic, Inc. (2011) Cardiac rhythm disease management product performance report: important patient management information for physicians. First edition, issue 64. Available at: http://wwwp.medtronic.com/productperformance-files/Issue64_MDT_CRDM_PPR_2011_1st_Edition.pdf
St. Jude Medical (2011) Product performance report: cardiac rhythm management. Available at: http://www.sjmprofessional.com/Resources/product-performance-reports/Product-Performance-Report.aspx
Boston Scientific (2011) CRM Product Performance Report Q2. Available at: http://www.bostonscientific.com/templatedata/imports/HTML/PPR/ppr/index.shtml
Biotronik. Product Performance Report (2011). Available at: http://www.biotronik.com/sixcms/media.php/162/ppr_january_2011.13959.pdf
Reynolds MR, Cohen DJ, Kugelmass AD, Brown PP, Becker ER, Culler SD et al (2006) The frequency and incremental cost of major complications among medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol 47:2493–2497
Hauser RG, Hayes DL, Epstein AE, Cannom DS, Vlay SC, Song SL et al (2006) Multicenter experience with failed and recalled implantable cardioverter-defibrillator pulse generators. Heart Rhythm 3(6):640–644
FDA. Medical & Radiation Emitting Device Recalls. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?start_search=1&event_id=28982 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?start_search=1&event_id=27417 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=907751
FDA. Medical & Radiation Emitting Device Recalls. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/resCollection_2.cfm?ID=39931&CREATE_DT=2005-06-30 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=40302 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=37338
Thijssen J, Borleffs CJ, Van Rees JB, Man S, De Bie MK, Venlet J, et al. (2011) Implantable cardioverter-defibrillator longevity under clinical circumstances: an analysis according to device type, generation, and manufacturer. Heart Rhythm (Epub ahead of print)
Dabiri Abkenari L, Theuns DA, Valk SD, Van Belle Y, de Groot NM et al (2011) Clinical experience with a novel subcutaneous implantable defibrillator system in a single center. Clin Res Cardiol 100(9):737–744
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shafat, T., Baumfeld, Y., Novack, V. et al. Significant differences in the expected versus observed longevity of implantable cardioverter defibrillators (ICDs). Clin Res Cardiol 102, 43–49 (2013). https://doi.org/10.1007/s00392-012-0493-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-012-0493-6