Abstract
Background
Tracheo-oesophageal malformations result from disturbed foregut separation during early development. The notochord, a specialised embryonic structure, forms immediately adjacent to the dividing foregut. In the Adriamycin mouse model of oesophageal atresia, foregut and notochord abnormalities co-exist, and the site and severity of foregut malformations closely correlate to the position and extent of the notochord defects. Notochord and foregut abnormalities also co-exist in the Noggin Knockout mouse as well in a small number of human cases. The notochord is a source of powerful molecular signals during early embryogenesis, being particularly important for neural crest development. The influence of notochord signaling on the adjacent foregut is not known. The purpose of this study was to examine the impact of notochord manipulation on foregut separation using a robust 3D explant method for culturing isolated foregut which permits oeosphageal and tracheal formation in vitro.
Methods
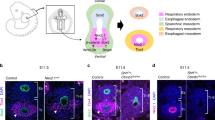
Foregut was micro-dissected from embryonic day 9 mice (License B100/4447 Irish Medicines Board), embedded in collagen and cultured for 48 h with native notochord intact (n = 6), notochord removed (n = 10) or additional notochord transplanted from stage matched controls (n = 8). Specimens were analysed for foregut morphology and molecular patterning using immunohistochemistry for Hnf3b (an endoderm marker) and Sox2 (a notochord and oesophageal marker) on cryosections.
Results
Foregut separation into distinct oesophagus and trachea was observed in isolated foregut specimens with or without their native notochord. In specimens with additional notochord transplants, foregut morphology and molecular patterning were comparable to controls whether or not the native notochord was maintained. In particular foregut separation was not disrupted by the transplantation of additional notochord at the dorsal foregut endoderm.
Conclusion
The relationship between the embryonic foregut and notochord is complex and ill-defined; however, the notochord does not contribute essentially to oesophagus and trachea formation beyond E9 in the mouse, and the transplantation of additional notochord does not disrupt foregut separation in 3D explant culture.





Similar content being viewed by others
References
van Straaten HW, Hekking JW, Wiertz-Hoessels EJ, Thors F, Drukker J (1988) Effect of the notochord on the differentiation of a floor plate area in the neural tube of the chick embryo. Anat Embryol (Berl) 177:317–324
Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP (1993) Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75:1417–1430
Marti E, Bumcrot DA, Takada R, McMahon AP (1995) Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature 375:322–325
Hebrok M, Kim SK, Melton DA (1998) Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev 12:1705–1713
Hajduk P, Sato H, Puri P, Murphy P (2011) Abnormal notochord branching is associated with foregut malformations in the adriamycin treated mouse model. PLoS One 6:e27635
Zorn AM, Wells JM (2009) Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol 25:221–251
Wells J, Melton D (1999) Vertebrate endoderm development. Annu Rev Cell Dev Biol 15:393–410
Li Y, Gordon J, Manley NR, Litingtung Y, Chiang C (2008) Bmp4 is required for tracheal formation: a novel mouse model for tracheal agenesis. Dev Biol 322:145–155
Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL (2007) Multiple dose dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134:2521–2531
Fausett SR, Brunet LJ, Klingensmith J (2014) BMP antagonism by Noggin is required in presumptive notochord cells for mammalian foregut morphogenesis. Dev Biol 391:111–124
Snider P, Simmons O, Rogers R, Young R, Gosnell M, Conway SJ (2011) Notochordal and foregut abnormalities correlate with elevated neural crest apoptosis in Patch embryos. Birth Defects Res A Clin Mol Teratol 91:551–564
Elliott GB, Tredwell SJ, Elliott KA (1970) The notochord as an abnormal organizer in production of congenital intestinal defect. Am J Roentgenol Radium Ther Nucl Med 110:628–634
Bentley JF, Smith JR (1960) Developmental posterior enteric remnants and spinal malformations: the split notochord syndrome. Arch Dis Child 35:76–86
Meller JL, Loeff DS, Reyes HM (1989) A variant of the split notochord syndrome. J Pediatr Surg 24:733–735
Tremblay KD, Zaret KS (2005) Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol 280:87–99
Desai TJ, Chen F, Lu J, Qian J, Niederreither K, Dolle P, Chambon P, Cardoso WV (2006) Distinct roles for retinoic acid receptors alpha and beta in early lung morphogenesis. Dev Biol 291:12–24
Theiler K (1989) The house mouse: atlas of embryonic development. Springer, Berlin, Heidelberg, New York
Ioannides AS, Massa V, Ferraro E, Cecconi F, Spitz L, Henderson DJ, Copp AJ (2010) Foregut separation and tracheo-oesophageal malformations: the role of tracheal outgrowth, dorso-ventral patterning and programmed cell death. Dev Biol 337:351–362
Dawrant MJ, Giles S, Bannigan J, Puri P (2007) Adriamycin mouse model: a variable but reproducible model of tracheo-oesophageal malformations. Pediatr Surg Int 23:469–472
Fan CM, Tessier-Lavigne M (1994) Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell 79:1175–1186
Hajduk P, May A, Puri P, Murphy P (2012) The effect of adriamycin exposure on the notochord of mouse embryos. Birth Defects Res B Dev Reprod Toxicol 95:175–183
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mc Laughlin, D., Murphy, P. & Puri, P. Notochord manipulation does not impact oesophageal and tracheal formation from isolated foregut in 3D explant culture. Pediatr Surg Int 32, 29–35 (2016). https://doi.org/10.1007/s00383-015-3809-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-015-3809-6