Abstract
Purpose
Human pigmented tissue-engineered skin substitutes represent an advanced therapeutic option to treat skin defects. The inflammatory response is one of the major factors determining integration and long-term survival of such a graft in vivo. The aim of the present study was to investigate the spatiotemporal distribution of host-derived macrophage and granulocyte graft infiltration as well as hypoxia-inducible factor 1 alpha (HIF-1-alpha) expression in a (nu/nu) rat model.
Methods
Keratinocytes, melanocytes, and fibroblasts derived from human skin biopsies were isolated, cultured, and expanded in vitro. Dermal fibroblasts were seeded into collagen type I hydrogels that were subsequently covered by keratinocytes and melanocytes in 5:1 ratio. These pigmented dermo-epidermal skin substitutes were transplanted onto full-thickness skin wounds on the back of immuno-incompetent rats and analyzed at early (1 and 3 weeks) and late (6 and 12 weeks) stages of wound healing. The expression of distinct inflammatory cell markers specific for granulocytes (HIS48) or macrophages (CD11b, CD68), as well as HIF-1-alpha were analyzed and quantified by immunofluorescence microscopy.
Results
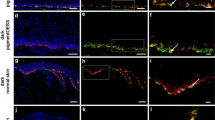
Our data demonstrate that granulocytes infiltrate the entire graft at 1 week post-transplantation. This was followed by monocyte/macrophage recruitment to the graft at 3–12 weeks. The macrophages were initially restricted to the borders of the graft (early stages), and were then found throughout the entire graft (late stages). We observed a time-dependent decrease of macrophages. Only a few graft-infiltrating granulocytes were found between 6–12 weeks, mostly at the graft borders. A heterogeneous expression of HIF-1-alpha was observed at both early and late wound healing stages.
Conclusions
Our findings demonstrate the spatiotemporal distribution of inflammatory cells in our transplants closely resembles the one documented for physiological wound healing.




Similar content being viewed by others
References
Loss M, Wedler V, Kunzi W, Meuli-Simmen C, Meyer VE (2000) Artificial skin, split-thickness autograft and cultured autologous keratinocytes combined to treat a severe burn injury of 93% of TBSA. Burns 26:644–652
Braziulis E, Diezi M, Biedermann T, Pontiggia L, Schmucki M, Hartmann-Fritsch F, Luginbuhl J, Schiestl C, Meuli M, Reichmann E (2012) Modified plastic compression of collagen hydrogels provides an ideal matrix for clinically applicable skin substitutes. Tissue Eng Part C-Methods 18:464–474
Klar AS, Guven S, Biedermann T, Luginbuhl J, Bottcher-Haberzeth S, Meuli-Simmen C, Meuli M, Martin I, Scherberich A, Reichmann E (2014) Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials 35:5065–5078
Klar AS, Bottcher-Haberzeth S, Biedermann T, Schiestl C, Reichmann E, Meuli M (2014) Analysis of blood and lymph vascularization patterns in tissue-engineered human dermo-epidermal skin analogs of different pigmentation. Pediatr Surg Int 30:223–231
Marino D, Luginbuhl J, Scola S, Meuli M, Reichmann E (2014) Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci Transl Med 6:221ra14
Bottcher-Haberzeth S, Klar AS, Biedermann T, Schiestl C, Meuli-Simmen C, Reichmann E, Meuli M (2013) “Trooping the color”: restoring the original donor skin color by addition of melanocytes to bioengineered skin analogs. Pediatr Surg Int 29:239–247
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453:314–321
Singer AJ, Clark RAF (1999) Mechanisms of disease- Cutaneous wound healing. N Engl J Med 341:738–746
Ross R, Odland G (1968) Human wound repair. 2. Inflammatory cells epithelial-mesenchymal interrelations and fibrogenesis. J Cell Biol 39:152–168
Ross R, Benditt EP (1962) Wound healing and collagen formation. 2. fine structure in experimental scurvy. J Cell Biol 12:533–551
Ross R, Benditt EP (1962) Wound healing and collagen formation. 3. A quantitative radioautographic study of utilization of proline-h3 in wounds from normal and scorbutic guinea pigs. J Cell Biol 15(1):99–108
Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S (1996) Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine 8:548–556
Nishio N, Okawa Y, Sakurai H, Isobe KI (2008) Neutrophil depletion delays wound repair in aged mice. Age 30:11–19
Ebaid H (2014) Neutrophil depletion in the early inflammatory phase delayed cutaneous wound healing in older rats: improvements due to the use of un-denatured camel whey protein. Diagn Pathol 4(9):46
Martin P, Leibovich SJ (2005) Inflammatory cells during wound, repair: the good, the bad and the ugly. Trends Cell Biol 15:599–607
Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA (2010) Differential roles of macrophages in diverse phases of skin repair. J Immunol 184:3964–3977
Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS (2003) HIF-1 alpha is essential for myeloid cell-mediated inflammation. Cell 112:645–657
Lewis JS, Lee JA, Underwood JCE, Harris AL, Lewis CE (1999) Macrophage responses to hypoxia, relevance to disease mechanisms. J Leukoc Biol 66:889–900
Semenza GL (2001) HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 13:167–171
Semenza GL (2001) Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med 7:345–350
Murdoch C, Giannoudis A, Lewis CE (2004) Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 104:2224–2234
Murdoch C, Muthana M, Lewis CE (2005) Hypoxia regulates macrophage functions in inflammation. J Immunol 175:6257–6263
Pontiggia L, Biedermann T, Meuli M, Widmer D, Bottcher-Haberzeth S, Schiestl C, Schneider J, Braziulis E, Montano I, Meuli-Simmen C, Reichmann E (2009) Markers to evaluate the quality and self-renewing potential of engineered human skin substitutes in vitro and after transplantation. J Invest Dermatol 129:480–490
Dovi JV, Szpaderska AM, DiPietro LA (2004) Neutrophil function in the healing wound: adding insult to injury? Thromb Haemost 92:275–280
Kim MH, Liu W, Borjesson DL, Curry FRE, Miller LS, Cheung AL, Liu FT, Isseroff RR, Simon SI (2008) Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J Invest Dermatol 128:1812–1820
Agaiby AD, Dyson M (1999) Immuno-inflammatory cell dynamics during cutaneous wound healing. J Anat 195:531–542
Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R (1998) Chemokines IL-8, GRO alpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol 153:1849–1860
Baskaran H, Yarmush ML, Berthiaume F (2000) Dynamics of tissue neutrophil sequestration after cutaneous burns in rats. J Surg Res 93:88–96
Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH (2011) Macrophages in skin injury and repair. Immunobiology 216:753–762
Rodero MP, Khosrotehrani K (2010) Skin wound healing modulation by macrophages. Int J Clin Exp Pathol 3:643–653
Brancato SK, Albina JE (2011) Wound macrophages as key regulators of repair origin, phenotype, and function. Am J Pathol 178:19–25
Adamson R (2009) Role of macrophages in normal wound healing: an overview. J Wound Care 18:349–351
Arnold F, West D, Kumar S (1987) Wound-healing- the effect of macrophage and tumor derived angiogenesis factors on skin-graft vascularization. Br J Exp Pathol 68:569–574
Crowther M, Brown NJ, Bishop ET, Lewis CE (2001) Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol 70:478–490
Ninikoski J, Heughan C, Hunt TK (1971) Oxygen and carbon dioxide tensions in experimental wounds. Surg Gynecol Obstet 133:1003–1007
Kivisaari J (1975) Oxygen and carbon-dioxide tensions in healing tissue. Acta Chir Scand 141:693–696
Kivisaari J, Niinikoski J (1975) Oxygen-tensions in healing anastomosis of rabbit aorta. Surgery 78:169–175
Acknowledgments
This work was financially supported by the EU-FP6 project EuroSTEC (soft tissue engineering for congenital birth defects in children: contract: LSHB-CT-2006-037409), by the EU-FP7 project EuroSkinGraft (FP7/2007-2013: grant agreement nr 279024), by the EU-FP7 (MultiTERM, grant agreement nr 238551), and the Clinical Research Priority Programs (CRPP) of the Faculty of Medicine of the University of Zurich. We are particularly grateful to the Gaydoul Foundation and the sponsors of “DonaTissue” (especially Thérèse Meier and Robert Zingg) for their generous financial support and interest in our work.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. S. Klar and S. Böttcher-Haberzeth authors contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Klar, A.S., Böttcher-Haberzeth, S., Biedermann, T. et al. Differential expression of granulocyte, macrophage, and hypoxia markers during early and late wound healing stages following transplantation of tissue-engineered skin substitutes of human origin. Pediatr Surg Int 30, 1257–1264 (2014). https://doi.org/10.1007/s00383-014-3616-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-014-3616-5