Abstract
Purpose
Human amniotic fluid comprises cells with high differentiation capacity, thus representing a potential cell source for skin tissue engineering. In this experimental study, we investigated the ability of human amniotic fluid derived cells to substitute dermal fibroblasts and support epidermis formation and stratification in a humanized animal model.
Methods
Dermo-epidermal skin grafts with either amniocytes or with fibroblasts in the dermis were compared in a rat model. Full-thickness skin wounds on the back of immuno-incompetent rats were covered with skin grafts with (1) amniocytes in the dermis, (2) fibroblasts in the dermis, or, (3) acellular dermis. Grafts were excised 7 and 21 days post transplantation. Histology and immunofluorescence were performed to investigate epidermis formation, stratification, and expression of established skin markers.
Results
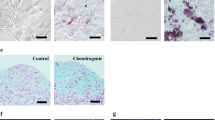
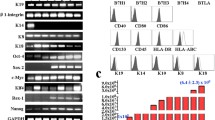
The epidermis of skin grafts engineered with amniocytes showed near-normal anatomy, a continuous basal lamina, and a stratum corneum. Expression patterns for keratin 15, keratin 16, and Ki67 were similar to grafts with fibroblasts; keratin 1 expression was not yet fully established in all suprabasal cell layers, expression of keratin 19 was increased and not only restricted to the basal cell layer as seen in grafts with fibroblasts. In grafts with acellular dermis, keratinocytes did not survive.
Conclusion
Dermo-epidermal skin grafts with amniocytes show near-normal physiological behavior suggesting that amniocytes substitute fibroblast function to support the essential cross-talk between mesenchyme and epithelia needed for epidermal stratification. This novel finding has considerable implications regarding tissue engineering.




Similar content being viewed by others
References
Kunisaki SM, Jennings RW, Fauza DO (2006) Fetal cartilage engineering from amniotic mesenchymal progenitor cells. Stem Cells Dev 15:245–253
Dobreva MP, Pereira PN, Deprest J, Zwijsen A (2010) On the origin of amniotic stem cells: of mice and men. Int J Dev Biol 54:761–777
Pappa KI, Anagnou NP (2009) Novel sources of fetal stem cells: where do they fit on the developmental continuum? Regen Med 4:423–433
Fauza D (2004) Amniotic fluid and placental stem cells. Best Pract Res Clin Obstet Gynaecol 18:877–891
Kaviani A, Perry TE, Dzakovic A, Jennings RW, Ziegler MM, Fauza DO (2001) The amniotic fluid as a source of cells for fetal tissue engineering. J Pediatr Surg 36:1662–1665
Mihu CM, Mihu D, Costin N, Rus Ciuca D, Susman S, Ciortea R (2008) Isolation and characterization of stem cells from the placenta and the umbilical cord. Rom J Morphol Embryol 49:441–446
In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C et al (2003) Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102:1548–1549
Yoon BS, Moon JH, Jun EK et al (2010) Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev 19:887–902
Kim J, Lee Y, Kim H et al (2007) Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif 40:75–90
Woodbury D, Kramer BC, Reynolds K, Marcus AJ, Coyne TM, Black IB (2006) Long-term cryopreserved amniocytes retain proliferative capacity and differentiate to ectodermal and mesodermal derivatives in vitro. Mol Reprod Dev 73:1463–1472
Fauza DO, Fishman SJ, Mehegan K, Atala A (1998) Videofetoscopically assisted fetal tissue engineering: bladder augmentation. J Pediatr Surg 33:7–12
Fauza DO, Marler JJ, Koka R, Forse RA, Mayer JE, Vacanti JP (2001) Fetal tissue engineering: diaphragmatic replacement. J Pediatr Surg 36:146–151
Fuchs JR, Terada S, Ochoa ER, Vacanti JP, Fauza DO (2002) Fetal tissue engineering: in utero tracheal augmentation in an ovine model. J Pediatr Surg 37:1000–1006 discussion 1000–1006
Fauza DO, Fishman SJ, Mehegan K, Atala A (1998) Videofetoscopically assisted fetal tissue engineering: skin replacement. J Pediatr Surg 33:357–361
Pontiggia L, Biedermann T, Meuli M et al (2009) Markers to evaluate the quality and self-renewing potential of engineered human skin substitutes in vitro and after transplantation. J Invest Dermatol 129:480–490
Biedermann T, Pontiggia L, Bottcher-Haberzeth S et al (2010) Human eccrine sweat gland cells can reconstitute a stratified epidermis. J Invest Dermatol 130:1996–2009
Böttcher-Haberzeth S, Biedermann T, Pontiggia L, et al. (2012) Human eccrine sweat gland cells turn into melanin-uptaking keratinocytes in dermo-epidermal skin substitutes. J Invest Dermatol. doi:10.1038/jid.2012.290
Costea DE, Loro LL, Dimba EAO, Vintermyr OK, Johannessen AC (2003) Crucial effects of fibroblasts and keratinocyte growth factor on morphogenesis of reconstituted human oral epithelium. J Invest Dermatol 121:1479–1486
Braziulis E, Diezi M, Biedermann T et al (2012) Modified plastic compression of collagen hydrogels provides an ideal matrix for clinically applicable skin substitutes. Tissue Eng Part C Methods 18:464–474
Armour AD, Powell HM, Boyce ST (2008) Fluorescein diacetate for determination of cell viability in tissue-engineered skin. Tissue Eng Part C 14:89–96
Bottcher-Haberzeth S, Biedermann T, Schiestl C et al (2012) Matriderm((R)) 1 mm versus Integra((R)) Single Layer 1.3 mm for one-step closure of full thickness skin defects: a comparative experimental study in rats. Pediatr Surg Int 28:171–177
Kiowski G, Biedermann T, Widmer DS et al (2012) Engineering melanoma progression in a humanized environment in vivo. J Invest Dermatol 132:144–153
Fusenig NE, Breitkreutz D, Dzarlieva RT, Boukamp P, Bohnert A, Tilgen W (1983) Growth and differentiation characteristics of transformed keratinocytes from mouse and human skin in vitro and in vivo. J Invest Dermatol 81:168s–175s
Lammers G, Verhaegen PD, Ulrich MM et al (2011) An overview of methods for the in vivo evaluation of tissue-engineered skin constructs. Tissue Eng Part B Rev 17:33–55
Rheinwald JG, Green H (1975) Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6:331–343
Leary T, Jones PL, Appleby M, Blight A, Parkinson K, Stanley M (1992) Epidermal keratinocyte self-renewal is dependent upon dermal integrity. J Invest Dermatol 99:422–430
Botchkarev VA, Kishimoto J (2003) Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc 8:46–55
Schultz GS, Wysocki A (2009) Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 17:153–162
Jauniaux E, Rodeck C (1995) Use, risks and complications of amniocentesis and chorionic villous sampling for prenatal diagnosis in early pregnancy. Early Pregnancy 1:245–252
Adzick NS, Thom EA, Spong CY et al (2011) A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364:993–1004
Meuli M, Moehrlen U, Flake AW, et al. (2012) Fetal Surgery in Zurich: Key Features of our First Open In Utero Repair of Myelomeningocele. Eur J Pediatr Surg (in press)
Zaulyanov L, Kirsner RS (2007) A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin Interv Aging 2:93–98
Marston WA (2004) Dermagraft, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Expert Rev Med Devices 1:21–31
Acknowledgments
This work was financially supported by the EU-FP6 project EuroSTEC (soft tissue engineering for congenital birth defects in children: contract: LSHB-CT-2006-037409), by the EU-FP7 project EuroSkinGraft (FP7/2007-2013: grant agreement n° 279024), and by the University of Zurich. We are particularly grateful to the Fondation Gaydoul and the sponsors of “DonaTissue” (Thérèse Meier and Robert Zingg) for their generous financial support and interest in our work.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hartmann-Fritsch, F., Hosper, N., Luginbühl, J. et al. Human amniotic fluid derived cells can competently substitute dermal fibroblasts in a tissue-engineered dermo-epidermal skin analog. Pediatr Surg Int 29, 61–69 (2013). https://doi.org/10.1007/s00383-012-3207-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-012-3207-2