Abstract
Purpose
Meningitis is relatively common in infants and young children and can cause permanent brain damage. The aim of this study was to determine whether meningitis is associated with fatty acids in cerebrospinal fluid (CSF).
Methods
CSF samples from children between 3 months and 6 years of age admitted to the Tabriz public hospitals who met clinical criteria of meningitis were collected at enrollment. A total of 81 samples were analyzed for fatty acid profile by gas–liquid chromatography.
Results
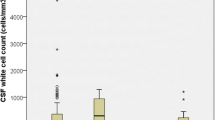
Children with a purulent meningitis demonstrated a higher percentage of oleic acid (p < 0.05, >10 %) and lower percentages of omega-3 polyunsaturated fatty acids (p < 0.001, <−40 %) than aseptic meningitis and nonmeningitis groups did. There was an inverse relationship between CSF long-chain omega-3 fatty acids and the total number of leukocytes and differential counts of neutrophils and lymphocytes in the purulent meningitis group. Moreover, significantly lower omega-3 fatty acids (p = 0.001, −37 %) and higher ratio of n-6/n-3 (p = 0.02, −29 %) were found in patients with purulent meningitis with sepsis than in those with meningitis and no sepsis.
Conclusions
This study provides evidence that purulent meningitis and its complication with sepsis are associated with important disturbances in CSF fatty acids, mainly deficiency in long-chain omega-3 polyunsaturated fatty acids.

Similar content being viewed by others
References
Pilitsis JG, Coplin WM, O’Regan MH, et al. (2003) Measurement of free fatty acids in cerebrospinal fluid from patients with hemorrhagic and ischemic stroke. Brain Res 985:198–201. doi:10.1016/S0006-8993(03)03044-0
Roelcke U, Heil J (1993) Fatty acids as markers of the blood-cerebrospinal fluid barrier function in man. Funct Neurol 8:189–192
Schmid SP, Schleicher ED, Cegan A, et al. (2012) Cerebrospinal fluid fatty acids in glucocerebrosidase-associated Parkinson’s disease. Mov Disord 27:288–293. doi:10.1002/mds.23984
Fonteh AN, Cipolla M, Chiang J, et al. (2014) Human cerebrospinal fluid fatty acid levels differ between supernatant fluid and brain-derived nanoparticle fractions, and are altered in Alzheimer’s disease. PLoS One. doi:10.1371/journal.pone.0100519
Jumpertz R, Guijarro A, Pratley RE, et al. (2012) Associations of fatty acids in cerebrospinal fluid with peripheral glucose concentrations and energy metabolism. PLoS One. doi:10.1371/journal.pone.0041503
Ghodoosifar S, Jafari-Rouhi AH, Pashapour A, et al. (2016) Correlation of secretory phospholipase-A2 activity and fatty acids in cerebrospinal fluid with liver enzymes tests. J Anal Res Clin Med 4:39–46. doi:10.15171/jarcm.2016.007
LaForce FM, Brice JL, Tornabene TG (1979) Diagnosis of bacterial meningitis by gas-liquid chromatography. II Analysis of spinal fluid J Infect Dis 140:453–464. doi:10.1093/infdis/140.4.453
French GL, Chan CY, Poon D, et al. (1990) Rapid diagnosis of bacterial meningitis by the detection of a fatty acid marker in CSF with gas chromatography-mass spectrometry and selected ion monitoring. J Med Microbiol 31:21–26. doi:10.1099/00222615-31-1-21
Zamir I, Grushka E, Cividalli G (1991) High-performance liquid chromatographic analysis of free palmitic and stearic acids in cerebrospinal fluid. J Chromatogr B Biomed Sci Appl 565:424–429. doi:10.1016/0378-4347(91)80404-Z
Saka HA, Thompson JW, Chen Y-S, et al. (2015) Chlamydia trachomatis infection leads to defined alterations to the lipid droplet proteome in epithelial cells. PLoS One 10:e0124630. doi:10.1371/journal.pone.0124630
Burth P, Younes-Ibrahim M, Santos MCB, et al. (2005) Role of nonesterified unsaturated fatty acids in the pathophysiological processes of leptospiral infection. J Infect Dis 191:51–57. doi:10.1086/426455
Hyvärinen K, Tuomainen AM, Laitinen S, et al. (2009) Chlamydial and periodontal pathogens induce hepatic inflammation and fatty acid imbalance in apolipoprotein E-deficient mice. Infect Immun 77:3442–3449. doi:10.1128/IAI.00389-09
World Health Organization WH (1998) Control of epidemic meningococcal disease: WHO practical guidelines.
Lentner C, Corporation C-G, Limited C-G (1981) Geigy scientific tables: physical chemistry, composition of blood, hematology, somatometric data, 8th ed. Medical Education Division, Ciba-Geigy Corporation, Basel
Levy MM, Fink MP, Marshall JC, et al. (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 29:530–538. doi:10.1007/s00134-003-1662-x
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120
Kruger PS (2009) Forget glucose: what about lipids in critical illness? Crit Care Resusc 11:305–309
Carpentier YA, Scruel O (2002) Changes in the concentration and composition of plasma lipoproteins during the acute phase response. Curr Opin Clin Nutr Metab Care 5:153–158
Seyer A, Boudah S, Broudin S, et al. (2016) Annotation of the human cerebrospinal fluid lipidome using high resolution mass spectrometry and a dedicated data processing workflow. Metabolomics 12:1–14. doi:10.1007/s11306-016-1023-8
Wishart DS, Lewis MJ, Morrissey JA, et al. (2008) The human cerebrospinal fluid metabolome. J Chromatogr B 871:164–173. doi:10.1016/j.jchromb.2008.05.001
Pilitsis JG, Coplin WM, O’Regan MH, et al. (2003) Free fatty acids in cerebrospinal fluids from patients with traumatic brain injury. Neurosci Lett 349:136–138. doi:10.1016/S0304-3940(03)00803-6
Farias SE, Heidenreich KA, Wohlauer MV, et al. (2011) Lipid mediators in cerebral spinal fluid of traumatic brain injured patients. J Trauma 71:1211–1218. doi:10.1097/TA.0b013e3182092c62
Vilanova JM, Figueras-Aloy J, Roselló J, et al. (1998) Arachidonic acid metabolites in CSF in hypoxic-ischaemic encephalopathy of newborn infants. Acta Paediatr 87:588–592. doi:10.1111/j.1651-2227.1998.tb01509.x
Kondo M, Kawai K, Yagyu K, et al. (2001) Changes in the cell structure of Flavobacterium psychrophilum with length of culture. Microbiol Immunol 45:813–818. doi:10.1111/j.1348-0421.2001.tb01320.x
Novák F, Borovská J, Vecka M, et al. (2010) [Alterations in fatty acid composition of plasma and erythrocyte lipids in critically ill patients during sepsis]. Cas Lek Cesk 149:324–331
Bazinet RP, Layé S (2014) Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci 15:771–785. doi:10.1038/nrn3820
Chen CT, Green JT, Orr SK, Bazinet RP (2008) Regulation of brain polyunsaturated fatty acid uptake and turnover. Prostaglandins Leukot Essent Fatty Acids 79:85–91. doi:10.1016/j.plefa.2008.09.003
Lachance C, Segura M, Dominguez-Punaro MC, et al. (2014) Deregulated balance of omega-6 and omega-3 polyunsaturated fatty acids following infection by the zoonotic pathogen Streptococcus suis. Infect Immun 82:1778–1785. doi:10.1128/IAI.01524-13
Das UN, Ramos EJB, Meguid MM (2003) Metabolic alterations during inflammation and its modulation by central actions of omega-3 fatty acids. Curr Opin Clin Nutr Metab Care 6:413–419. doi:10.1097/01.mco.0000078981.18774.5e
Thors VS, Þórisdóttir A, Erlendsdóttir H, et al. (2004) The effect of dietary fish oil on survival after infection with Klebsiella pneumoniae or Streptococcus pneumoniae. Scand J Infect Dis 36:102–105. doi:10.1080/00365540310018914
Acknowledgments
We are grateful to all of the subjects who kindly agreed to participate in this study. The authors would like to thank Maryam Darabi, PhD (Institute of Clinical Chemistry, Zurich), for her critical reading of the manuscript. This study was partially conducted as part of a Master’s thesis project no. 94/1-2/2 at the Tabriz University of Medical Sciences for the first author. This work was supported by the Pediatric Health Research Center at the Tabriz University of Medical Sciences [grant number 5/93/140]. Support was also provided by the Emergency Medicine Research Team at Tabriz University of Medical Sciences.
Authors’ contribution
Conceived and designed study: MD and MB. Performed measurements: EE, AM, MS, and SG. Analyzed the data: EE, MB, and AM. Contributed reagents/materials: EE, AM, MS, SG, and AA. Wrote the paper: MD, MB, EE, AM, MS, AA and SG. Critically revised the manuscript: MD and MB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ᅟ
This cross-sectional study was reviewed and approved by the ethics committee of Tabriz University of Medical Sciences (IRB permission number 5.4.11707), and a direct parent (mother or father) provided written informed consent. This study was performed according with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This study was partially conducted as part of a Master’s thesis project no. 94/1-2/2 at the Tabriz University of Medical Sciences for the first author. This work was supported by the Pediatric Health Research Center at the Tabriz University of Medical Sciences [grant number 5/93/140]. Support was also provided by the Emergency Medicine Research Team at Tabriz University of Medical Sciences.
Rights and permissions
About this article
Cite this article
Ekhtiyari, E., Barzegar, M., Mehdizadeh, A. et al. Differential fatty acid analysis of cerebrospinal fluid in infants and young children with suspected meningitis. Childs Nerv Syst 33, 111–117 (2017). https://doi.org/10.1007/s00381-016-3232-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-016-3232-x