Abstract
Purpose
In pediatric neurosurgery, decompressive craniectomy and correction of congenital cranial anomalies can result in major cranial defects. Corrective cranioplasty for the repair of these critical-sized defects is not only a cosmetic issue. The limited availability of suitable autogenous bone and the morbidity of donor site harvesting have driven the search for new approaches with biodegradable and bioactive materials. This study aimed to assess the healing of rabbit calvarial critical-sized defects filled with osteogenic material, either with bioactive glass scaffolds or tricalcium phosphate granules in various combinations with adipose stem cells or bone marrow stem cells, BMP-2, BMP-7, or VEGF to enhance osteogenesis.
Methods
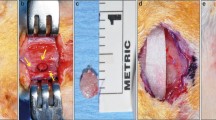
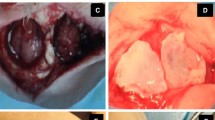
Eighty-two bicortical full thickness critical-sized calvarial defects were operated. Five defects were left empty as negative control defects. The remaining 77 defects were filled with solid bioactive glass scaffolds or tricalcium phosphate granules seeded with adipose or bone marrow derived stem cells in combination with BMP-2, BMP-7, or VEGF. The defects were allowed to heal for 6 weeks before histologic and micro-CT analyses.
Results
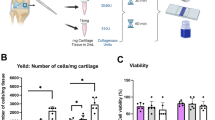
Micro-CT examination at the 6-week post-operative time point revealed that defects filled with stem cell-seeded tricalcium phosphate granules resulted in new bone formation of 6.0 %, whereas defects with bioactive glass scaffolds with stem cells showed new bone formation of 0.5 to 1.7 %, depending on the growth factor used.
Conclusions
This study suggests that tricalcium phosphate granules combined with stem cells have osteogenic potential superior to solid bioactive glass scaffolds with stem cells and growth factors.




Similar content being viewed by others
References
Martin KD, Franz B, Kirsch M et al (2014) Autologous bone flap cranioplasty following decompressive craniectomy is combined with a high complication rate in pediatric traumatic brain injury patients. Acta Neurochir 156:813–824
Piitulainen JM, Kauko T, Aitasalo KM et al (2015) Outcomes of cranioplasty with synthetic materials and autologous bone grafts. World Neurosurg 83:708–714
Rish BL, Dillon JD, Meirowsky AM et al (1979) Cranioplasty: a review of 1030 cases of penetrating head injury. Neurosurgery 4:381–385
Sanan A, Haines SJ (1979) Repairing holes in the head: a history of cranioplasty. Neurosurgery 40:588–603
Sándor GK, Tuovinen VJ, Wolff J et al (2013) Adipose stem cell (ASC) tissue engineered construct used to treat large anterior mandibular defect: a case report and review of the clinical application of GMP-level ASCs for bone regeneration. J Oral Maxillofac Surg 71:938–950
Ardjomandi N, Duttenhoefer F, Xavier S et al (2015) In vivo comparison of hard tissue regeneration with ovine mesenchymal stem cells processed with either the FICOLL method or the BMAC method. J Craniomaxillofac Surg 43:1177–1183
Feroze AH, Walmsley GG, Choudhri O et al (2015) Evolution of cranioplasty techniques in neurosurgery: historical review, pediatric considerations, and current trends. J Neurosurg 20:1–10
Serlo WS, Ylikontiola LP, Lähdesluoma N et al (2011) Posterior cranial vault distraction osteogenesis in craniosynostosis: estimated increases in intracranial volume. Childs Nerv Syst 27:627–633
Wellisz T, Dougherty W, Gross J (1992) Craniofacial applications for the Medpor porous polyethylene flexblock implant. J Craniofac Surg 3:101–107
Moreira-Gonzalez A, Jackson IT, Miyawaki T et al (2003) Clinical outcome in cranioplasty: critical review in long-term follow-up. J Craniofac Surg 14:144–153
Vallittu PK (2014) High-aspect ratio fillers: fiber-reinforced composites and their anisotropic properties. Dent Mater 31:1–7
Thesleff T, Lehtimäki K, Niskakangas T et al (2011) Cranioplasty with adipose-derived stem cells and biomaterial: a novel method for cranial reconstruction. Neurosurgery 68:1535–1540
Stieglitz L, Fung C, Murek M et al (2015) What happens to the bone flap; Long-term outcome after reimplantation of cryoconserved bone flaps in a consecutive series of 92 patients. Acta Neurochir 157:275–280
Sundseth J, Sundseth A, Berg-Johnsen J et al (2014) Cranioplasty with autologous cryopreserved bone after decompressive craniectomy. Complications and risk factors for developing surgical site infection. Acta Neurochir 156:805–811
Velardi F, Amante PR, Caniglia M et al (2006) Osteogenesis induced by autologous bone marrow cells transplant in the pediatric skull. Childs Nerv Syst 22:1158–66
Mesimäki K, Lindroos B, Törnwall J et al (2009) Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg 38:201–209
Sándor GK, Numminen J, Wolff J et al (2014) Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl Med 3:530–540
Berger M, Probst F, Schwartz C et al (2015) A concept for scaffold-based tissue engineering in alveolar cleft osteoplasty. J Cranio-Maxillofac Surg 43:830–836
Leucht P, Helms JA (2015) Wnt signaling: an emerging target for bone regeneration. J Am Acad Orthop Surg 23:67–68
Jan A, Sándor GK, Iera D et al (2006) Hyperbaric oxygen results in an increase in rabbit calvarial critical sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endodont 101:144–149
Fok TC, Jan A, Peel SA et al (2008) Hyperbaric oxygen results in an increase in vascular endothelial growth factor (VEGF) protein expression in rabbit calvarial critical sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endodont 105:417–422
Lappalainen OP, Korpi R, Haapea M et al (2015) Healing of rabbit calvarial critical-sized defects using autogenous bone grafts and fibrin glue. Childs Nerv Syst 31:581–7
Tirkkonen L, Haimi S, Huttunen S et al (2013) Osteogenic medium is superior to growth factors in differentiation of human adipose stem cells towards bone-forming cells in 3D culture. Eur Cell Mater 25:144–158
Jan A, Sándor GK, Brkovic BM et al (2009) Effects of hyperbaric oxygen on grafted and non-grafted on calvarial critical-sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endodont 107:157–163
Jan A, Sándor GK, Brkovic BM et al (2010) Effects of hyperbaric oxygen on demineralized bone matrix and biphasic calcium phosphate bone substitutes. Oral Surg Oral Med Oral Pathol Oral Radiol Endodont 109:60–67
Frassanito P, Tamburrini G, Massimi L et al (2015) Post-marketing surveillance of Custom Bone Service implanted in children under 7 years old. Acta Neurochirurg (Wein) 157:115–121
Waselau M, Patrikoski M, Juntunen M et al. (2012) Effects of bioactive glass S53P4 or beta tricalcium phosphate and Bone Morphogenetic Proteins 2 and BMP-7 on osteogenic differentiation of human adipose stem cells. J Tissue Engineering doi:2041731412467789
Bess S, Line BG, Lafage V et al (2014) Does recombinant human bone morphogenetic protein-2 use in adult spinal deformity increase complications and are complications associated with location of rhBMP-2 use? A prospective, multicenter study of 279 consecutive patients. Spine (Phila Pa 1976) 39:233–242
Devine JG, Dettori JR, France JC et al (2012) Brodt E, McGuire RA. The use of rhBMP in spine surgery: is there a cancer risk? Evid Based Spine Care J 3:35–41
Carragee EJ, Chu G, Rohatgi R et al (2013) Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am 95:1537–1545
Skovrlj B, Koehler SM, Anderson PA et al (2015) Association between BMP-2 and carcinogenicity. Spine (Phila Pa 1976) 40:1862–1871
Acknowledgments
The authors wish to express their sincere gratitude for the financial support provided to this project by the Emil Aaltonen Foundation, Tampere, Finland, the ITI Foundation, Basel, Switzerland (ITI grant number 619–2009), the University of Oulu EVO and VTR Grant Funds, the Academy of Finland (grants number. 268378 and 273571), Sigrid Juselius Foundation and European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement no. 336267.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The following animal care and experimental protocol received ethical approvals (Decision ESHL-2008-07701/Ym-23) from the Oulu University Hospital Ethical Committee. The study was performed in accordance with the Helsinki Declaration and its later amendments.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lappalainen, OP., Karhula, S., Haapea, M. et al. Bone healing in rabbit calvarial critical-sized defects filled with stem cells and growth factors combined with granular or solid scaffolds. Childs Nerv Syst 32, 681–688 (2016). https://doi.org/10.1007/s00381-016-3017-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-016-3017-2