Abstract
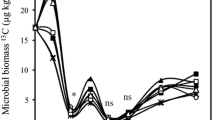
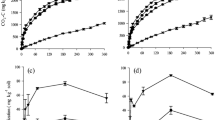
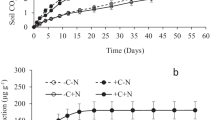
Crop residue quality and quantity have contrasting effects on soil organic matter (SOM) decomposition, but the mechanisms explaining such priming effect (PE) are still elusive. To reveal the role of residue quality and quantity in SOM priming, we applied two rates (5.4–10.8 g kg−1) of 13C-labeled wheat residues (separately: leaves, stems, roots) to soil and incubated for 120 days. To distinguish PE mechanisms, labeled C was traced in CO2 efflux and in microbial biomass and enzyme activities (involved in C, N, and P cycles) were measured during the incubation period. Regardless of residue type, PE intensity declined with increasing C additions. Roots were least mineralized but caused up to 60% higher PE compared to leaves or stems. During intensive residue mineralization (first 2–3 weeks), the low or negative PE resulted from pool substitution. Thereafter (15–60 days), a large decline in microbial biomass along with increased enzyme activity suggested that microbial necromass served as SOM primer. Finally, incorporation of SOM-derived C into remaining microbial biomass corresponded to increased enzyme activity, which is indicative of SOM cometabolism. Both PE and enzyme activities were primarily correlated with residue-metabolizing soil microorganisms. A unifying model demonstrated that PE was a function of residue mineralization, with thresholds for strong PE increase of up to 20% root, 44% stem, and 51% leaf mineralization. Thus, root mineralization has the lowest threshold for a strong PE increase. Our study emphasizes the role of residue-feeding microorganisms as active players in the PE, which are mediated by quality and quantity of crop residue additions.






Similar content being viewed by others
References
Aber JD, Melillo JM (1982) Nitrogen immobilization in decaying hardwood leaf litter as a function of initial nitrogen and lignin content. Can J Bot 60:2263–2269. doi:10.1139/b82-277
Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, Burke IC, Hart SC (2008) Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob Chang Biol 14:2636–2660. doi:10.1111/j.1365-2486.2008.01674.x
Berg B, McClaugherty C (2014) Decomposition as a process: some main features. In: Berg B, McClaugherty C (eds) Plant litter — decomposition, humus formation, carbon sequestration, Third Edition Edn. Springer, Heidelberg, New York, Dordrecht, London, pp. 11–34. doi:10.1007/978-3-642-38821-7_2
Bertrand I, Chabbert B, Kurek B, Recous S (2006) Can the biochemical features and histology of wheat residues explain their decomposition in soil? Plant Soil 281:291–307. doi:10.1007/s11104-005-4628-7
Blagodatskaya Е, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131. doi:10.1007/s00374-008-0334-y
Blagodatskaya E, Kuzyakov Y (2013) Active microorganisms in soil: critical review of estimation criteria and approaches. Soil Biol Biochem 67:192–211. doi:10.1016/j.soilbio.2013.08.024
Blagodatskaya E, Yuyukina T, Blagodatsky S, Kuzyakov Y (2011a) Three-source-partitioning of microbial biomass and of CO2 efflux from soil to evaluate mechanisms of priming effects. Soil Biol Biochem 43:778–786. doi:10.1016/j.soilbio.2010.12.011
Blagodatskaya E, Yuyukina T, Blagodatsky S, Kuzyakov Y (2011b) Turnover of soil organic matter and of microbial biomass under C3-C4 vegetation change: consideration of 13C fractionation and preferential substrate utilization. Soil Biol Biochem 43:159–166. doi:10.1016/j.soilbio.2010.09.028
Blagodatskaya E, Khomyakov N, Myachina O, Bogomolova I, Blagodatsky S, Kuzyakov Y (2014) Microbial interactions affect sources of priming induced by cellulose. Soil Biol Biochem 74:39–49. doi:10.1016/j.soilbio.2014.02.017
Blagodatsky S, Blagodatskaya E, Yuyukina T, Kuzyakov Y (2010) Model of apparent and real priming effects: linking microbial activity with soil organic matter decomposition. Soil Biol Biochem 42:1275–1283. doi:10.1016/j.soilbio.2010.04.005
Bromand S, Whalen J, Janzen H (2001) A pulse-labelling method to generate 13C-enriched plant materials. Plant Soil 235:253–257. doi:10.1023/A:1011922103323
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234. doi:10.1016/j.soilbio.2012.11.009
Castellano MJ, Mueller KE, Olk DC, Sawyer JE, Six J (2015) Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob Chang Biol 21:3200–3209. doi:10.1111/gcb.12982
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob Chang Biol 20:2356–2367. doi:10.1111/gcb.12475
Chen S, Wang Y, Hu Z, Gao H (2015) CO2 emissions from a forest soil as influenced by amendments of different crop straws: implications for priming effects. Catena 131:56–63. doi:10.1016/j.catena.2015.03.016
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Chang Biol 19:988–995. doi:10.1111/gcb.12113
Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix ML, Wall DH, Parton WJ (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci 8:776–779. doi:10.1038/ngeo2520
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–848. doi:10.1016/S0038-0717(03)00123-8
Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280. doi:10.1038/nature06275
Garcia-Pausas J, Paterson E (2011) Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon. Soil Biol Biochem 43:1705–1713. doi:10.1016/j.soilbio.2011.04.016
Guenet B, Neill C, Bardoux G, Abbadie L (2010) Is there a linear relationship between priming effect intensity and the amount of organic matter input? Appl Soil Ecol 46:436–442. doi:10.1016/j.apsoil.2010.09.006
Hayes JM (2004) An introduction to isotopic calculations. Woods Hole, MA 02543, USA: http://www.whoi.edu/cms/files/jhayes/2005/9/IsoCalcs30Sept04_5183.pdf.
Horvath RS (1972) Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriol Rev 36:146–155
Hoyle FC, Murphy DV, Brookes PC (2008) Microbial response to the addition of glucose in low-fertility soils. Biol Fertil Soils 44:571–579. doi:10.1007/s00374-007-0237-3
Jenkinson DS, Fox RH, Rayner JH (1985) Interactions between fertilizer nitrogen and soil nitrogen—the so-called ‘priming’ effect. J Soil Sci 36:425–444. doi:10.1111/j.1365-2389.1985.tb00348.x
Jiang-shan Z, Jian-fen G, Guang-shui C, Wei Q (2005) Soil microbial biomass and its controls. J Forest Res 16:327–330. doi:10.1007/BF02858201
Johnson JMF, Novak JM, Varvel GE, Stott DE, Osborne SL, Karlen DL, Lamb JA, Baker J, Adler PR (2014) Crop residue mass needed to maintain soil organic carbon levels: can it be determined? BioEnergy Res 7:481–490. doi:10.1007/s12155-013-9402-8
Kramer S, Marhan S, Ruess L, Armbruster W, Butenschoen O, Haslwimmer H, Kuzyakov Y, Pausch J, Scheunemann N, Schoene J, Schmalwasser A (2012) Carbon flow into microbial and fungal biomass as a basis for the belowground food web of agroecosystems. Pedobiologia 55:111–119. doi:10.1016/j.pedobi.2011.12.001
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371. doi:10.1016/j.soilbio.2010.04.003
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498. doi:10.1016/S0038-0717(00)00084-5
Ladd JN, Foster R, Nannipieri P, Oades JM (1996) Soil structure and biological activity. In: Stotzky G, Bollag J-M (eds) Soil biochemistry 9. Marcel Dekker, New York, pp 23–78
Lehmann J, Kleber M (2015) The contentious nature of soil organic matter. Nature 528:60–68. doi:10.1038/nature16069
Leifeld J, von Lützow M (2014) Chemical and microbial activation energies of soil organic matter decomposition. Biol Fertil Soils 50:147–153. doi:10.1007/s00374-013-0822-6
Lian T, Wang G, Yu Z, Li Y, Liu X, Jin J (2016) Carbon input from 13C-labelled soybean residues in particulate organic carbon fractions in a Mollisol. Biol Fertil Soils 52:331–339. doi:10.1007/s00374-015-1080-6
Makarov MI, Malysheva TI, Menyailo OV, Soudzilovskaia NA, Van Logtestijn RSP, Cornelissen JHC (2015) Effect of K2SO4 concentration on extractability and isotope signature (δ13C and δ15N) of soil C and N fractions. Eur J Soil Sci 66:417–426. doi:10.1111/ejss.12243
Meyer SL (1975) Data analysis for scientists and engineers. Wiley, New York
Miltner A, Kindler R, Knicker H, Richnow HH, Kästner M (2009) Fate of microbial biomass-derived amino acids in soil and their contribution to soil organic matter. Org Geochem 40:978–985. doi:10.1016/j.orggeochem.2009.06.008
Miltner A, Bombach P, Schmidt-Brücken B, Kästner M (2012) SOM genesis: microbial biomass as a significant source. Biogeochemistry 111:41–55. doi:10.1007/s10533-011-9658-z
Nannipieri P, Johnson RL, Paul EA (1978) Criteria for measurement of microbial growth and activity in soil. Soil Biol Biochem 10:223–229. doi:10.1016/0038-0717(78)90100-1
Nannipieri P, Kandeler E, Ruggiero P (2002) Enzyme activities and microbiological and biochemical processes in soil. In: Burns RG, Dick RP (eds) Enzymes in the environment. Activity, ecology and applications. Marcel Dekker, New York, pp 1–33
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bunemann EK, Obreson A, Frossard E (eds) Phosphorus in action, Soil Biology, vol 26. Springer Verlag, Berlin Heidelberg, pp 215–243
Nannipieri P, Giagnoni L, Renella G, Puglisi E, Ceccanti B, Masciandaro G, Fornasier F, Moscatelli MC, Marinari S (2012) Soil enzymology: classical and molecular approaches. Biol Fertil Soils 48:743–762. doi:10.1007/s00374-012-0723-0
Nguyen TT, Marschner P (2016) Soil respiration, microbial biomass and nutrient availability in soil after repeated addition of low and high C/N plant residues. Biol Fertil Soils 52:165–176. doi:10.1007/s00374-015-1063-7
Nottingham AT, Griffiths H, Chamberlain PM, Stott AW, Tanner EVJ (2009) Soil priming by sugar and leaf-litter substrates: a link to microbial groups. Appl Soil Ecol 42:183–190. doi:10.1016/j.apsoil.2009.03.003
Paterson E, Sim A (2013) Soil-specific response functions of organic matter mineralization to the availability of labile carbon. Glob Chang Biol 19:1562–1571. doi:10.1111/gcb.12140
Poirier V, Angers D, Rochette P, Whalen J (2013) Initial soil organic carbon concentration influences the short-term retention of crop-residue carbon in the fine fraction of a heavy clay soil. Biol Fertil Soils 49:527–535. doi:10.1007/s00374-013-0794-6
Pritsch K, Raidl S, Marksteiner E, Blaschke H, Agerer R, Schloter M, Hartmann A (2004) A rapid and highly sensitive method for measuring enzyme activities in single mycorrhizal tips using 4-methylumbelliferone-labelled fluorogenic substrates in a microplate system. J Microbiol Meth 58:233–241. doi:10.1016/j.mimet.2004.04.001
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356. doi:10.1007/s11104-004-0907-y
Sanaullah M, Razavi BS, Blagodatskaya E, Kuzyakov Y (2016) Spatial distribution and catalytic mechanisms of β-glucosidase activity at the root-soil interface. Biol Fertil Soils 52:505–514. doi:10.1007/s00374-016-1094-8
Schnecker J, Wild B, Hofhansl F, Alves RJ, Bárta J, Čapek P, Fuchslueger L, Gentsch N, Gittel A, Guggenberger G, Hofer A (2014) Effects of soil organic matter properties and microbial community composition on enzyme activities in cryoturbated arctic soils. PLoS One 9:e94076. doi:10.1371/journal.pone.0094076
Shahbaz M, Kuzyakov Y, Heitkamp F (2016a) Decrease of soil organic matter stabilization with increasing inputs: mechanisms and controls. Geoderma. doi:10.1016/j.geoderma.2016.05.019
Shahbaz M, Kuzyakov Y, Maqsood S, Wendland M, Heitkamp F (2016b) Decadal nitrogen fertilization decreases mineral-associated and subsoil carbon: a 32 year study. Land Degrad Develop. doi:10.1002/ldr.2667
Stewart CE, Moturi P, Follett RF, Halvorson AD (2015) Lignin biochemistry and soil N determine crop residue decomposition and soil priming. Biogeochemistry 124:335–351. doi:10.1007/s10533-015-0101-8
Tian J, Pausch J, Yu G, Blagodatskaya E, Gao Y, Kuzyakov Y (2015) Aggregate size and their disruption affect 14C-labeled glucose mineralization and priming effect. Appl Soil Ecol 90:1–10. doi:10.1016/j.apsoil.2015.01.014
Vance E, Brookes P, Jenkinson D (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. doi:10.1016/0038-0717(87)90052-6
Wagner GH (1968) Significance of microbial tissue to soil organic matter. In: Isotopes and radiation in soil organic matter studies. FAO/IAEA, Technical meeting, Vienna, pp 197–205
Wang H, Boutton T, Xu W, Hu G, Jiang P, Bai E (2015) Quality of fresh organic matter affects priming of soil organic matter and substrate utilization patterns of microbes. Sci Rep 5:10102. doi:10.1038/srep10102
Wang J, Dokohely ME, Xiong Z, Kuzyakov Y (2016) Contrasting effects of aged and fresh biochars on glucose-induced priming and microbial activities in paddy soil. J Soils Sediments 16:191–203. doi:10.1007/s11368-015-1189-0
Webster R (2007) Analysis of variance, inference, multiple comparisons and sampling effects in soil research. Eur J Soil Sci 58:74–82. doi:10.1111/j.1365-2389.2006.00801.x
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction—an automated procedure. Soil Biol Biochem 22:1167–1169. doi:10.1016/0038-0717(90)90046-3
Xiao C, Guenet B, Zhou Y, Su J, Janssens IA (2015) Priming of soil organic matter decomposition scales linearly with microbial biomass response to litter input in steppe vegetation. Oikos 124:649–657. doi:10.1111/oik.01728
Xu X, Ouyang H, Richter A, Wanek W, Cao G, Kuzyakov Y (2011) Spatio-temporal variations determine plant-microbe competition for inorganic nitrogen in an alpine meadow. J Ecol 99:563–571. doi:10.1111/j.1365-2745.2010.01789.x
Acknowledgements
We acknowledge the financial support provided by the German Academic Exchange Service (DAAD) to MS and Alexander von Humboldt Foundation (AvH) to MSU. EB’s participation was supported by the Russian Science Foundation (project N 14-14-00625). We also acknowledge the technical support of Klaus Schützenmeister in isotope labeling of the plant material. We are thankful to Karin Schmidt and Anita Kriegel for laboratory assistance. The isotopic analyses were performed at the Kompetenzzentrum Stabile Isotope (KOSI), Goettingen. This study was funded by the Deutsche Forschungsgemeinschaft (DFG, projects HE 6726/6 and KU 1184/29).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahbaz, M., Kuzyakov, Y., Sanaullah, M. et al. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: mechanisms and thresholds. Biol Fertil Soils 53, 287–301 (2017). https://doi.org/10.1007/s00374-016-1174-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-016-1174-9