Abstract
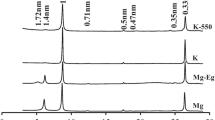
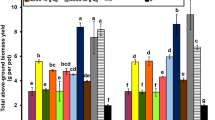
A pot experiment was carried out under greenhouse conditions with canola plants growing in a mixture of quartz (as the filling materials), glauconitic shale, and silicate dissolving bacteria. During a period of 180 days, pots were irrigated with K (+K) or without K (−K). At the end of the growing period, plants were harvested and their K uptake determined by flame photometer following dry ash extraction. The clay-sized particles in each pot were studied by X-ray diffractometer. Vermiculization of glauconite occurred with both nutrient solutions. The intensity of the 14 Å peak in the treatment with silicate dissolving bacteria and K-free nutrient solution was higher than with the complete nutrient solution. Under the treatment with silicate dissolving bacteria and K-free nutrient solution the 14/10 Å peak ratio was 7.58 and it was statistically different at 95 % level compared to the peaks of the other treatments. Weathering of mica by K release from mica interlayers and the formation of vermiculite were the major process occurring in the rhizosphere. The results of mineralogical studies are therefore in line with the findings of greenhouse experiment both confirming that the application of glauconite can be beneficial to plant mainly through K release from mineral weathering even in a single growth period.







Similar content being viewed by others
References
Amorosia A, Sammartinoa I, Tateob F (2007) Evolution patterns of glaucony maturity: a mineralogical and geochemical approach. Deep-Sea Res 54:1364–1374
Badr MA (2006) Efficiency of K-feldspar combined with organic materials and silicate dissolving bacteria on tomato yield. J Appl Sci Res 2:1191–1198
Badr MA, Shafei AM, Sharaf El-Deen SH (2006) The dissolution of K and phosphorus bearing minerals by silicate dissolving bacteria and their effect on sorghum growth. J Agric Biol Sci 2:5–11
Bakhshandeh S, Khormali F, Dordipour E, Olamaie M, Kehl M (2011) Comparing the weathering of soil and sedimentary palygorskite in the rhizosphere zone. Appl Clay Sci 54:235–241
Balland-Bolou-Bi C, Poszwa A (2012) Effect of calco-magnesian amendment on the mineral weathering abilities of bacterial communities in acidic and silicate-rich soils. Soil Biol Biochem 50:108–117
Barker WW, Welch SA, Welch SC, Banfield F (1998) Experimental observations of the effects of bacteria on aluminosilicate weathering. Am Mineral 83:1551–1563
Barré P, Velde B, Catel N, Abbadie L (2007) Soil–plant potassium transfer: impact of plant activity on clay minerals as seen from X-ray diffraction. Plant Soil 292:137–146
Bennett PC, Rogers JR, Choi WJ (2001) Silicates, silicate weathering, and microbial Ecology. Geomicrobiol J 18:3–19
Bullock P, Federoff N, Jongerius A, Stoops G, Tursina T, Babel U (1985) Handbook for soil thin section description. Waine Research Publications, Wolverhampton
Calvaruso C, Turpault MP, Frey-Klett P (2006) Root-associated bacteria contribute to mineral weathering and to mineral nutrition in trees: a budgeting analysis. Appl Environ Microbiol 72:1258–1266
Carey PL, Metherell AK (2003) Rates of release of non-exchangeable potassium in New Zealand soils measured by a modified sodium tetraphenyl-boron method. N Z J Agric Res 46:185–197
Colombo C, Palumbo G, He JZ, Pinton R, Cesco S (2013) Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J Soils Sediments 14:538–548
Fang SX, Yan LH (2006) Solubilization of potassium bearing minerals by wild type strain of Bacillus edaphicus and its mutants and increased potassium by wheat. Can J Microbiol 52:66–72
Fanning DS, Keramidas VZ, El-desoky MAA (1989) Micas. In: Dixon JB, Weed SB (eds) Minerals in soil environments. SSSA Publishing, Madison, WI, pp 551–634
Gamalero E, Martinotti MG, Trotta A, Lemanceau P, Berta G (2002) Morphological modifications induced by Pseudomonas fluorescens and Glomus mosseae in the root system of tomato differ according to plant growth conditions. New Phytol 155:293–300
Grayston SJ, Vaughan D, Jones D (1996) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol 5:29–56
Hinsinger P (2002) Potassium. In: Lal R (ed) Encyclopedia of soil science. Marcel Dekker, Inc., New York, pp 1035–1039
Hinsinger P, Jaillard B (1993) Root-induced release of interlayer potassium and vermiculitization of phlogopite as related to potassium depletion in the rhizosphere of ryegrass. J Soil Sci 44:525–534
Hinsinger P, Jaillard B, Dufey JE (1992) Rapid weathering of a trioctahedral mica by the roots of ryegrass. Soil Sci Soc Am J 56:977–982
Hinsinger P, Elsass F, Jaillard B, Robert M (1993) Root-induced irreversible transformation of a trioctahedral mica in rhizosphere of rape. J Soil Sci 44:535–545
Jackson ML (1975) Soil Chemical Analysis. Madison, WI, USA
Jalali M (2007) Spatial variability in potassium release among calcareous soils of western Iran. Geoderma 140:42–51
Khademi H, Arocena JM (2008) Kaolinite formation from palygorskite and sepiolite in rhizosphere soils. Clays Clay Miner 56:422–436
Khayamim F, Khademi H, Sabzalian MR (2010) Effect of Neotyphodium endophyte-tall fescue symbiosis on mineralogical changes in clay-sized phlogopite and muscovite. Plant Soil 341:473–484
Khormali F, Ghergherechi S, Kehl M, Ayoubi S (2012) Soil formation in loess-derived soils along a subhumid to humid climate gradient, Northeastern Iran. Geoderma 180:113–122
Khormali F, Rezaei F, Rahimzadeh N, Hosseinifard SJ, Dordipour E (2015) Rhizosphere-induced weathering of minerals in loess-derived soils of Golestan Province, Iran. Geoderma Regional 5:34–43
Kittrick JA, Hope EW (1963) A procedure for the particle size separation of soils for X-ray diffraction analysis. Soil Sci 96:312–325
Marie-Pierre T, Claude N, Christophe C (2009) Rhizosphere impact on the dissolution of test minerals in a forest ecosystem. Geoderma 153:147–154
Martin HW, Sparks DL (1985) On the behavior of nonexchangeable potassium in soils. Commun Soil Sci Plant Anal 16:133–162
McLean EO, Watson ME (1985) Soil measurements of plant available potassium. In: Munson RD (ed) Potassium in Agriculture. SSSA Publishing, Madison, pp 278–309
Mehra OP, Jackson ML (1960) Iron oxide removal from soils and clays by a dithionite citrate system with sodium bicarbonate. Clays Clay Miner 7:317–327
Mengel K, Schubert S (1985) Active extrusion of protons into deionized water by roots of intact maize plants. Plant Physiol 79:344–350
Mengel K, Uhlenbecker K (1993) Determination of available interlayer potassium and its uptake by ryegrass. Soil Sci Soc Am J 57:761–766
Mimmo T, Del Buono D, Terzano R, Tomasi N, Vigani G, Crecchio C, Pinton R, Zocchi G, Cesco S (2014) Rhizospheric organic compounds in the soil–microorganism–plant system: their role in iron availability. Eur J Soil Sci 65:629–642
Moritsuka N, Yanai J, Kosaki T (2004) Possible processes releasing non-exchangeable potassium from the rhizosphere of maize. Plant Soil 258:261–268
Naderizadeh Z, Khademi H, Arocena JM (2010) Organic matter induced mineralogical changes in clay-sized phlogopite and muscovite in alfalfa rhizosphere. Geoderma 159:296–303
Norouzi S, Khademi H (2010) Ability of alfalfa (Medicago sativa L.) to take up potassium from different micaceous minerals and consequent vermiculitization. Plant Soil 328:83–93
Pii Y, Cesco S, Mimmo T (2015a) Shoot ionome to predict the synergism and antagonism between nutrients as affected by substrate and physiological status. Plant Physiol Biochem 94:48–56
Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C (2015b) Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Boil Fertil Soils 51:403–415
Robert M, Berthelin J (1986) Role of biological and biochemical factors in soil mineral weathering. In: Huang PM, Schnitzer M (eds) Interactions of soil minerals with natural organics and microbes. SSSA Publishing, Madison, pp 453–495
Rogers JR, Bennett PC (2004) Mineral stimulation of subsurface microorganisms: release of limiting nutrients from silicates. Chem Geol 203:91–108
Sheng XF, He LY (2006) Solubilization of potassium bearing minerals by a wild type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Can J Microbiol 52:66–72
Sheng XF, Huang WY (2002) Study on the conditions of potassium release by strain NBT of silicate bacteria. Sci Agric Sin 35:673–677
Sheng XF, Xia JJ, Chen J (2003) Mutagenesis of the Bacillus edaphicus strain NBT and its effect on growth of chilli and cotton. Agric Sci China 2:40–412
Sparks DL, Huang PM (1985) Physical chemistry of soil potassium. In: Munson RD (ed) Potassium in agriculture. SSSA Publishing, Madison, pp 201–276
Springob G, Richter J (1998) Measuring interlayer potassium release rates from soil minerals. II. A percolation procedure to study the influence of the variable ‘solute K’ in the <1–10μM range. Z Pflanzenernähr Bodenkunde 161:315–322
Steffens D, Sparks DL (1997) Kinetics of nonexchangeable ammonium release from soils. Soil Sci Soc Am J 61:455–462
Stegner R (2002) Plant nutrition studies. Lamotte, M.A, USA
Stoops G (2003) Guidelines for the analysis and description of soil and regolith thin sections. SSSA Publishing, Madison
Stoops G, Marcelino V, Mees F (2010) Interpretation of micromorphological features of soils and regoliths. Elsevier Science Publishing
Styriakova I, Styriak I, Galko I, Hradil D, Bezdicka P (2003) The release of iornbearing minerals and dissolution of feldspar by heterotrophic bacteria of Bacillus species. Ceram Silik 47:20–26
Sugumaran P, Janarthanam B (2007) Solubilization of potassium containing minerals by bacteria and their effect on plant growth. World J Agric Sci 3:350–355
Terzano R, Cesco S, Mimmo T (2014) Dynamics, thermodynamics and kinetics of exudates: crucial issues in understanding rhizosphere processes. Plant Soil 386:399–406
Terzano R, Cuccovillo G, Eliana Gattullo C, Medici L, Tomasi N, Pinton R, Mimmo T, Cesco S (2015) Combined effect of organic acids and flavonoids on the mobilization of major and trace elements from soil. Biol Fertil Soils. doi:10.1007/s00374-015-1009-0
Tifac (2001) Techno market survey on technologies for agricultural applications of glauconite, a potash mineral. TIFAC report. http://tifac.org.in/index.php?option=com_content&view=article&id=729&Itemid=205.
Tributh H, von Boguslawski E, van Lieres A, Steffens D, Mengel K (1987) Effect of potassium removal by crops on transformation of illitic clay minerals. Soil Sci 143:404–409
Vandevivere P, Welch SA, Ullman WJ, Kirchman DJ (1994) Enhanced dissolution of silicate minerals by bacteria at near neutral pH. Microb Ecol 27:241–251
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahimzadeh, N., Khormali, F., Olamaee, M. et al. Effect of canola rhizosphere and silicate dissolving bacteria on the weathering and K release from indigenous glauconite shale. Biol Fertil Soils 51, 973–981 (2015). https://doi.org/10.1007/s00374-015-1043-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-015-1043-y