Abstract
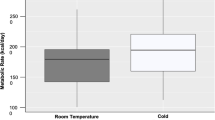
Obligate hibernators, such as ground squirrels, display circannual patterns which persist even under constant laboratory conditions, suggesting that they are regulated by endogenous rhythms. Brown adipose tissue (BAT) is important for thermogenesis during periodic arousals from hibernation when core body temperature rises spontaneously from 5 to 37 °C. In most small eutherians BAT growth requires several weeks of cold exposure. We hypothesized that in the thirteen-lined ground squirrel (Ictidomys tridecemlineatus), a hibernator, BAT growth is regulated, in part, by an endogenous rhythm and we predicted that this growth would precede the hibernation season without cold exposure. We tested this prediction using repeated water–fat magnetic resonance imaging over a year, including the hibernation season. Thoracic BAT depots increased in volume from spring through autumn even though animals were housed at ~22 °C. Subsequent cold exposure (5 °C) enlarged the thoracic BAT further. The fat fraction of this tissue fell significantly during the period of peak growth, indicating relative increases in non-triglyceride components, perhaps mitochondria or vasculature. We also found that the proportion of the body consisting of white adipose tissue (WAT) increased steadily from spring through autumn, and fell throughout hibernation, mirroring changes in body mass. Unlike BAT, WAT fat fractions remained constant (near 90%) throughout the year. Future studies will evaluate the significance of photoperiod and cold exposure on the growth of these tissues. We also found tissue with a fat fraction characteristic of BAT in the head near the eyes, a potentially novel discovery that requires further confirmation.





Similar content being viewed by others
References
Armitage KB, Shulenberger E (1972) Evidence for a circannual metabolic cycle in Citellus tridecemlineatus, a hibernator. Comp Biochem Physiol A Physiol 42:667–688
Bagchi M, Kim LA, Boucher J et al (2013) Vascular endothelial growth factor is important for brown adipose tissue development and maintenance. FASEB J Off Publ Fed Am Soc Exp Biol 27:3257–3271. doi:10.1096/fj.12-221812
Ballinger MA, Hess C, Napolitano MW, et al (2016) Seasonal changes in brown adipose tissue mitochondria in a mammalian hibernator: from gene expression to function. Am J Physiol Regul Integr Comp Physiol. doi:10.1152/ajpregu.00463.2015
Carey FG (1982) A brain heater in the swordfish. Science 216:1327–1329
Feist et al (1985) Regulation of energy stores in arctic ground squirrels: brown fat thermogenic capacity, lipoprotein lipase and pancreatic hormones during fat deposition. In: Heller HC, Musaacchia XJ, Wang LCH (eds) Living in the cold: physiological and biochemical adaptations. Elsevier Science Publishing Co., Inc., New York pp 281–285
Geiser F (2004) Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol 66:239–274. doi:10.1146/annurev.physiol.66.032102.115105
Guglielmo CG, McGuire LP, Gerson AR, Seewagen CL (2011) Simple, rapid, and non-invasive measurement of fat, lean, and total water masses of live birds using quantitative magnetic resonance. J Ornithol 152:75. doi:10.1007/s10336-011-0724-z
Hampton M, Melvin RG, Andrews MT (2013) Transcriptomic analysis of brown adipose tissue across the physiological extremes of natural hibernation. PLOS One 8:e85157. doi:10.1371/journal.pone.0085157
Himms-Hagen J (1985) Brown adipose tissue metabolism and thermogenesis. Annu Rev Nutr 5:69–94. doi:10.1146/annurev.nu.05.070185.000441
Hindle AG, Martin SL (2014) Intrinsic circannual regulation of brown adipose tissue form and function in tune with hibernation. Am J Physiol Endocrinol Metab 306:E284–E299
Hines CDG, Yu H, Shimakawa A et al (2010) Quantification of hepatic steatosis with 3-T MR imaging: validation in ob/ob mice. Radiology 254:119–128. doi:10.1148/radiol.09090131
Hochachka PW (1974) Regulation of heat production at the cellular level. Fed Proc 33:2162–2169
Hu HH, Smith DL, Nayak KS et al (2010) Identification of brown adipose tissue in mice with fat-water IDEAL-MRI. J Magn Reson Imaging JMRI 31:1195–1202. doi:10.1002/jmri.22162
Laursen WJ, Mastrotto M, Pesta D et al (2015) Neuronal UCP1 expression suggests a mechanism for local thermogenesis during hibernation. Proc Natl Acad Sci USA 112:1607–1612. doi:10.1073/pnas.1421419112
McFarlane SV, Mathers KE, Staples JF (in press) Reversible temperature-dependent alterations of brown adipose tissue mitochondrial respiration during torpor in a mammalian hibernator. Am J Physiol R doi:10.1152/ajpregu.00316.2016
Nedergaard J, Cannon B (1984) Preferential utilization of brown adipose tissue lipids during arousal from hibernation in hamsters. Am J Physiol Regul Integr Comp Physiol 247:R506–R512
Nizielski SE, Billington CJ, Levine AS (1989) Brown fat GDP binding and circulating metabolites during hibernation and arousal. Am J Physiol Regul Integr Comp Physiol 257:R536–R541
Oelkrug R, Polymeropoulos ET, Jastroch M (2015) Brown adipose tissue: physiological function and evolutionary significance. J Comp Physiol B 185:587–606
Prakash KNB, Verma SK, Yaligar J et al (2016) Segmentation and characterization of interscapular brown adipose tissue in rats by multi-parametric magnetic resonance imaging. Magn Reson Mater Phys Biol Med 29:277–286. doi:10.1007/s10334-015-0514-3
Puigserver P, Wu Z, Park CW et al (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839
Rafael J, Vsiansky P, Heldmaier G (1985a) Seasonal adaptation of brown adipose tissue in the Djungarian hamster. J Comp Physiol B 155:521–528. doi:10.1007/BF00684683
Rafael J, Vsiansky P, Heldmaier G (1985b) Increased contribution of brown adipose tissue to nonshivering thermogenesis in the Djungarian hamster during cold-adaptation. J Comp Physiolv B 155:717–722.
Rasmussen JM, Entringer S, Nguyen A, et al (2013) Brown adipose tissue quantification in human neonates using water-fat separated MRI. PLOS One 8:e77907. doi:10.1371/journal.pone.0077907
Reeder SB, Robson PM, Yu H et al (2009) Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging JMRI 29:1332–1339. doi:10.1002/jmri.21751
Reeder SB, Hu HH, Sirlin CB (2012) Proton density fat-fraction: a standardized mr-based biomarker of tissue fat concentration. J Magn Reson Imaging 36:1011–1014
Romu T, Elander L, Leinhard OD et al (2015) Characterization of brown adipose tissue by water–fat separated magnetic resonance imaging. J Magn Reson Imaging 42:1639–1645. doi:10.1002/jmri.24931
Runcie RM, Dewar H, Hawn DR et al (2009) Evidence for cranial endothermy in the opah (Lampris guttatus). J Exp Biol 212:461–470. doi:10.1242/jeb.022814
Russell RL, O’Neill PH, Epperson LE, Martin SL (2010) Extensive use of torpor in thirteen-lined ground squirrels in the fall prior to cold exposure. J Comp Physiolv B 180:1165–1172. doi:10.1007/s00360-010-0484-8
Sepulveda CA, Dickson KA, Frank LR, Graham JB (2007) Cranial endothermy and a putative brain heater in the most basal tuna species, Allothunnus fallai. J Fish Biol 70:1720–1733. doi:10.1111/j.1095-8649.2007.01446.x
Sheriff MJ, Fridinger RW, Tøien Ø et al (2013) Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol Biochem Zool 86:515–527
Sun W, Uchida K, Takahashi N et al (2016) Activation of TRPV2 negatively regulates the differentiation of mouse brown adipocytes. Pflüg Arch Eur J Physiol 468:1527–1540
Wang LCH (1979) Time patterns and metabolic rates of natural torpor in the Richardson’s ground squirrel. Can J Zool 57:149–155. doi:10.1139/z79-012
Ward JM, Armitage KB (1981) Circannual rhythms of food consumption, body mass, and metabolism in yellow-bellied marmots. Comp Biochem Physiol A Physiol 69:621–626
Wu J, Cohen P, Spiegelman BM (2013) Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev 27:234–250
Yan J, Burman A, Nichols C et al (2006) Detection of differential gene expression in brown adipose tissue of hibernating arctic ground squirrels with mouse microarrays. Physiol Genom 25:346–353. doi:10.1152/physiolgenomics.00260.2005
Yu H, Shimakawa A, McKenzie CA et al (2008) Multi-echo water-fat separation and simultaneous R2* estimation with multi-frequency fat spectrum modeling. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med 60:1122–1134. doi:10.1002/mrm.21737
Acknowledgements
We thank Katherine Mathers, Sarah McFarlane and Natalie Po for help with animal care and project development. This study was supported financially by the Natural Sciences and Engineering Research Council through Discovery Grants (JFS; RGPIN-2014-04860, CAM RGPIN- 2013-356310) and the Canada Research Chairs program (CAM 950-228038).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Breukelen.
This manuscript is part of the special issue Hibernation—Guest Editors: Frank van Breukelen and Jenifer C. Utz.
Electronic supplementary material
Below is the link to the electronic supplementary material.
360_2017_1075_MOESM1_ESM.tif
Fig. S1 Volume segmentation for total body and specific tissue types. For each image slice the total animal area was outlined manually (in red) using the T1-weighted images (a). Using the T2-weighted images the area of white adipose tissue, with fat fractions greater than 80 (b; highlighted in red) and brown adipose tissue with fat fractions between 25 and 70 (c; outlined in red) were determined for each slice. Using the slice thickness of 0.9mm the volume of each region of interest was calculated and summed for the entire animal (TIF 607 KB)
360_2017_1075_MOESM2_ESM.tif
Fig. S2 Water-fat MRI reveals a tissue within the head (highlighted in red), near the eye, that has a fat fraction resembling BAT. These images were collected from the same animal and suggest that volume of this tissue changes throughout the year (TIF 2614 KB)
360_2017_1075_MOESM3_ESM.tif
Fig. S3 Relative change in the quantity of thoracic brown adipose tissue over a year. For each individual the value at each time point is expressed relative to the first data point collected in June 2015. Data are presented as mean ± SE, n = 3. The green arrow indicates the date ambient temperature started to be reduced 1°C/day from ~22oC and light blue arrow indicate the date at which the temperature reached 5°C, corresponding largely with the first hibernation bouts (TIF 5199 KB)
Rights and permissions
About this article
Cite this article
MacCannell, A., Sinclair, K., Friesen-Waldner, L. et al. Water–fat MRI in a hibernator reveals seasonal growth of white and brown adipose tissue without cold exposure. J Comp Physiol B 187, 759–767 (2017). https://doi.org/10.1007/s00360-017-1075-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1075-8