Abstract
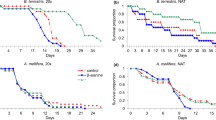
Many dilute nectars consumed by bird pollinators contain secondary metabolites, potentially toxic chemicals produced by plants as defences against herbivores. Consequently, nectar-feeding birds are challenged not only by frequent water excess, but also by the toxin content of their diet. High water turnover, however, could be advantageous to nectar consumers by enabling them to excrete secondary metabolites or their transformation products more easily. We investigated how the alkaloid nicotine, naturally present in nectar of Nicotiana species, influences osmoregulation in white-bellied sunbirds Cinnyris talatala and Cape white-eyes Zosterops virens. We also examined the metabolic fate of nicotine in these two species to shed more light on the post-ingestive mechanisms that allow nectar-feeding birds to tolerate nectar nicotine. A high concentration of nicotine (50 µM) decreased cloacal fluid output and increased its osmolality in both species, due to reduced food intake that led to dehydration. White-eyes excreted a higher proportion of the ingested nicotine-containing diet than sunbirds. However, sugar concentration did not affect nicotine detoxification and elimination. Both species metabolised nicotine, excreting very little unchanged nicotine. Cape white-eyes mainly metabolised nicotine through the cotinine metabolic pathway, with norcotinine being the most abundant metabolite in the excreta, while white-bellied sunbirds excreted mainly nornicotine. Both species also utilized phase II conjugation reactions to detoxify nicotine, with Cape white-eyes depending more on the mercapturic acid pathway to detoxify nicotine than white-bellied sunbirds. We found that sunbirds and white-eyes, despite having a similar nicotine tolerance, responded differently and used different nicotine-derived metabolites to excrete nicotine.



Similar content being viewed by others
References
Adler LS (2000) The ecological significance of toxic nectar. Oikos 91:409–420
Adler LS, Seifert MG, Wink M, Morse GE (2012) Reliance on pollinators predicts defensive chemistry across tobacco species. Ecol Lett 15:1140–1148. doi:10.1111/j.1461-0248.2012.01838.x
Almeida D, Maldonado E, Imran Khan I et al (2016) Whole-genome identification, phylogeny, and evolution of the cytochrome P450 family 2 (CYP2) subfamilies in birds. Genome Biol Evol 8(4):1115–1131. doi:10.1093/gbe/evw041
Au J, Marsh KJ, Wallis IR, Foley WJ (2013) Whole-body protein turnover reveals the cost of detoxification of secondary metabolites in a vertebrate browser. J Comp Physiol B 183:993–1003. doi:10.1007/s00360-013-0754-3
Bakken BH, Sabat P (2006) Gastrointestinal and renal responses to water intake in the green-backed firecrown (Sephanoides sephanoides), a South American hummingbird. Am J Regul Integr Comp Physiol 291:R830–R386
Baldwin IT (2001) An ecologically motivated analysis of plant-herbivore interactions in native tobacco. Plant Physiol 127:1449–1458. doi:10.1104/pp.010762
Barceló G, Ríos JM, Maldonado K, Sabat P (2016) Energetic costs and implications of the intake of plant secondary metabolites on digestive and renal morphology in two austral passerines. J Comp Physiol B 186:625–637. doi:10.1007/s00360-016-0974-4
Beuchat CA, Calder III WA, Braun EJ (1990) The integration of osmoregulation and energy balance in hummingbirds. Physiol Zool 1059–1081
Burn JH, Truelove LH, Burn I (1945) Antidiuretic action of nicotine. Br Med J 1:403
Caviedes-Vidal E, McWhorter TJ, Lavin SR, et al (2007) The digestive adaptation of flying vertebrates: high intestinal paracellular absorption compensates for smaller guts. Proc Natl Acad Sci 104:19132–19137
Chen G, Giambrone NE, Dluzen DF et al (2010) Glucuronidation genotypes and nicotine metabolic phenotypes: importance of functional UGT2B10 and UGT2B17 polymorphisms. Cancer Res 70:7543–7552. doi:10.1158/0008-5472.CAN-09-4582
De Souza EC, Silva Jr MRE (1977) The release of vasopressin by nicotine: further studies on its site of action. J Physiol 265:297–311
Dearing MD, Mangione AM, Karasov WH (2001) Plant secondary compounds as diuretics: an overlooked consequence. Am Zool 41:890–901
Dearing MD, Mangione AM, Karasov WH (2002) Ingestion of plant secondary compounds causes diuresis in desert herbivores. Oecologia 130:576–584
Dearing MD, Foley WJ, McLean S (2005) The influence of plant secondary metabolites on the nutritional ecology of herbivorous terrestrial vertebrates. Annu Rev Ecol Evol Syst 36:169–189. doi:10.1146/annurev.ecolsys.36.102003.152617
del Rio CM, Schondube JE, McWhorter TJ, Herrera LG (2001) Intake responses in nectar feeding birds: digestive and metabolic causes, osmoregulatory consequences, and coevolutionary effects. Am Zool 41:902–915
Dieckhaus CM, Fernández-Metzler CL, King R et al (2005) Negative ion tandem mass spectrometry for the detection of glutathione conjugates. Chem Res Toxicol 18:630–638
Du Rand EE, Smit S, Beukes M, et al (2015) Detoxification mechanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine. Sci Rep 5:11779
Fleming PA, Nicolson SW (2003) Osmoregulation in an avian nectarivore, the whitebellied sunbird Nectarinia talatala: response to extremes of diet concentration. J Exp Biol 206:1845–1854. doi:10.1242/jeb.00351
Fleming PA, Gray DA, Nicolson SW (2004a) Osmoregulatory response to acute diet change in an avian nectarivore: rapid rehydration following water shortage. Comp Biochem Physiol A 138:321–326. doi:10.1016/j.cbpb.2004.04.003
Fleming PA, Gray DA, Nicolson SW (2004b) Circadian rhythm of water balance and aldosterone excretion in the whitebellied sunbird Nectarinia talatala. J Comp Physiol [B] 174:341–346. doi:10.1007/s00360-004-0419-3
Fossi MC, Massi A, Lari L et al (1995a) Interspecies differences in mixed function oxidase activity in birds: relationship between feeding habits, detoxfication activities and organochlorine accumulation. Environ Pollut 90:15–24
Fossi MC, Massi A, Leonzio C et al (1995b) Interspecific differences in mixed function oxidase activity in birds: a tool to identify “species at risk”. Sci Total Environ 171:221–226
Geerts S, Pauw A (2009) African sunbirds hover to pollinate an invasive hummingbird-pollinated plant. Oikos 118:573–579. doi:10.1111/j.1600-0706.2009.17167.x
Gloss AD, Vassao DG, Hailey AL et al (2014) Evolution in an ancient detoxification pathway is coupled with a transition to herbivory in the Drosophilidae. Mol Biol Evol 31:2441–2456. doi:10.1093/molbev/msu201
Gray DA, Fleming PA, Nicolson SW (2004) Dietary intake effects on arginine vasotocin and aldosterone in cloacal fluid of whitebellied sunbirds (Nectarinia talatala). Comp Biochem Physiol A 138:441–449. doi:10.1016/j.cbpb.2004.06.006
Green AK, Barnes DM, Karasov WH (2005) A new method to measure intestinal activity of P-glycoprotein in avian and mammalian species. J Comp Physiol B 175:57–66. doi:10.1007/s00360-004-0462-0
Guglielmo CG, Karasov WH, Jakubas (1996) Nutritional costs of a plant secondary metabolite explain selective foraging by Ruffed Grouse. Ecology 77:1103–1115
Hukkanen J, Jacob P III, Benowitz NL (2005) Metabolism and disposition kinetics of nicotine. Pharmacol Rev 57:79–115. doi:10.1124/pr.57.1.3
Jakubas WJ, Karasov WH, Guglielmo CG (1993) Ruffed Grouse tolerance and biotransformation of the plant secondary metabolite coniferyl benzoate. Condor 95:625–640
Johnson SD, Nicolson SW (2008) Evolutionary associations between nectar properties and specificity in bird pollination systems. Biol Lett 4:49–52
Karasov WH (2011) Digestive physiology: a view from molecules to ecosystem. Am J Regul Integr Comp Physiol 301:R276–R284. doi:10.1152/ajpregu.00600.2010
Karasov WH, Martínez del Rio C (2007) Physiological ecology: how animals process energy, nutrients, and toxins. Princeton University Press, Princeton
Karasov WH, Caviedes-Vidal E, Bakken BH et al (2012) Capacity for absorption of water-soluble secondary metabolites greater in birds than in rodents. PLoS ONE 7:e32417. doi:10.1371/journal.pone.0032417
Kessler D, Diezel C, Baldwin IT (2010) Changing pollinators as a means of escaping herbivores. Curr Biol 20:237–242. doi:10.1016/j.cub.2009.11.071
Kessler D, Bhattacharya S, Diezel C et al (2012) Unpredictability of nectar nicotine promotes outcrossing by hummingbirds in Nicotiana attenuata: variability in nectar nicotine promotes outcrossing. Plant J 71:529–538. doi:10.1111/j.1365-313X.2012.05008.x
Kohl KD, Pitman E, Robb BC et al (2015) Monoterpenes as inhibitors of digestive enzymes and counter-adaptations in a specialist avian herbivore. J Comp Physiol B 185:425–434. doi:10.1007/s00360-015-0890-z
Lerch-Henning S, Nicolson SW (2013) Bird pollinators differ in their tolerance of a nectar alkaloid. J Avian Biol 44:408–416. doi:10.1111/j.1600-048X.2013.00079.x
Lerch-Henning S, Nicolson SW (2015) Effects of nicotine on the digestive performance of nectar-feeding birds reflect their relative tolerance to this alkaloid. Comp Biochem Physiol A 190:47–53. doi:10.1016/j.cbpa.2015.08.015
Lessner KM, Dearing MD, Izhaki I et al (2015) Small intestinal hydrolysis of plant glucosides: higher glucohydrolase activities in rodents than passerine birds. J Exp Biol 218:2666–2669. doi:10.1242/jeb.121970
Liska DJ (1998) The detoxification enzyme systems. Altern Med Rev 3:187–198
Liukkonen-Anttila T, Honkanen H, Peltokangas P et al (2003) Cytochrome P450 enzyme activity in five herbivorous, non-passerine bird species. Comp Biochem Physiol Part C Toxicol Pharmacol 134:69–77
Lushchak VI (2012) Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 2012:1–26. doi:10.1155/2012/736837
Mangione AM, Dearing MD, Karasov WH (2004) Creosote bush (Larrea tridentata) resin increases water demands and reduces energy availability in desert woodrats (Neotoma lepida). J Chem Ecol 30:1409–1429
McWhorter TJ, del Rio CM (1999) Food ingestion and water turnover in hummingbirds: how much dietary water is absorbed? J Exp Biol 202:2851–2858
McWhorter TJ, del Rio CM, Pinshow B (2003) Modulation of ingested water absorption by Palestine sunbirds: evidence for adaptive regulation. J Exp Biol 206:659–666
Meger M, Meger-Kossien I, Schuler-Metz A et al (2002) Simultaneous determination of nicotine and eight nicotine metabolites in urine of smokers using liquid chromatography–tandem mass spectrometry. J Chromatogr B 778:251–261
Miller EI, Norris H-RK, Rollins DE et al (2010) A novel validated procedure for the determination of nicotine, eight nicotine metabolites and two minor tobacco alkaloids in human plasma or urine by solid-phase extraction coupled with liquid chromatography–electrospray ionization–tandem mass spectrometry. J Chromatogr B 878:725–737
Napier KR, Purchase C, McWhorter TJ et al (2008) The sweet life: diet sugar concentration influences paracellular glucose absorption. Biol Lett 4:530–533. doi:10.1098/rsbl.2008.0253
Napier KR, Fleming PA, McWhorter TJ (2014) Mistletoebirds and xylose: Australian frugivores differ in their handling of dietary sugars. Physiol Biochem Zool 87:445–455. doi:10.1086/675493
Nicolson SW, Fleming PA (2003) Energy balance in the whitebellied sunbird Nectarinia talatala: constraints on compensatory feeding, and consumption of supplementary water. Funct Ecol 17:3–9
Nicolson SW, Fleming PA (2014) Drinking problems on a “simple”diet: physiological convergence in nectar-feeding birds. J Exp Biol 217:1015–1023
Purchase C, Napier KR, Nicolson SW et al (2013a) Gastrointestinal and renal responses to variable water intake in whitebellied sunbirds and New Holland honeyeaters. J Exp Biol 216:1537–1545
Purchase C, Nicolson SW, Fleming PA (2013b) Salt intake and regulation in two passerine nectar drinkers: white-bellied sunbirds and New Holland honeyeaters. J Comp Physiol B 183:501–510
Rainio MJ, Kanerva M, Wahlberg N et al (2012) Variation of basal EROD activities in ten passerine bird species–relationships with diet and migration status. PLoS ONE 7:e33926. doi:10.1371/journal.pone.0033926
Rangiah K, Hwang W-T, Mesaros C, et al (2011) Nicotine exposure and metabolizer phenotypes from analysis of urinary nicotine and its 15 metabolites by LC–MS. Bioanalysis 3:745–761. doi:10.4155/bio.11.42
Rinaldi R, Eliasson E, Swedmark S, Morgenstern R (2002) Reactive intermediates and the dynamics of glutathione transferases. Drug Metab Dispos 30:1053–1058
Ríos JM, Mangione AM, Marone L (2012) Tolerance to dietary phenolics and diet breadth in three seed-eating birds: implications for graminivory. J Exp Zool Part Ecol Genet Physiol 317:425–433. doi:10.1002/jez.1735
Rooke IJ, Bradshaw SD, Langworthy RA (1983) Aspects of the water, electrolyte and carbohydrate physiology of the silvereye, Zosterops lateralis (Aves). Aust. J Zool 31:695–704
Sorensen JS, Dearing MD (2006) Efflux transporters as a novel herbivore counter mechanism to plant chemical defenses. J Chem Ecol 32:1181. doi:10.1007/s10886-006-9079-y
Sorensen JS, McLister JD, Dearing MD (2005) Plant secondary metabolites compromise the energy budgets of specialist and generalist mammalian herbivores. Ecology 86:125–139
Steppuhn A, Gase K, Krock B et al (2004) Nicotine’s defensive function in nature. PLoS Biol 2:e217. doi:10.1371/journal.pbio.0020217
Stevenson PC, Nicolson SW, Wright GA (2017) Plant secondary metabolites in nectar: impacts on pollinators and ecological functions. Funct Ecol. doi:10.1111/1365-2435.12761
Struempf HM, Schondube JE, Martínez del Rio C (1999) The cyanogenic glycoside amygdalin does not deter consumption of ripe fruit by cedar waxings. The Auk 116:749–758
Tadmor-Melamed H, Markman S, Arieli A et al (2004) Limited ability of Palestine sunbirds Nectarinia osea to cope with pyridine alkaloids in nectar of tree tobacco Nicotiana glauca. Funct Ecol 18:844–850
Acknowledgements
This research was funded by the University of Pretoria and the National Research Foundation (73671). We are grateful to Jan Cilliers Park and the Pretoria National Botanic Gardens for permission to mist-net sunbirds and white-eyes under permit from the Gauteng Directorate of Nature Conservation. All bird care procedure and experimental protocols followed the institutional regulations of the Animal Use and Care Committee of the University of Pretoria (reference number: EC022-09). We thank Dr. M. Stander of the Central Analytical Facility at Stellenbosch University for conducting the nicotine metabolite analyses, Prof. Z. Apostolides for assistance with analysis and Dr. F. Demares for advice on statistical procedure.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
Lerch-Henning, S., Du Rand, E.E. & Nicolson, S.W. Detoxification and elimination of nicotine by nectar-feeding birds. J Comp Physiol B 187, 591–602 (2017). https://doi.org/10.1007/s00360-016-1055-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-1055-4