Abstract
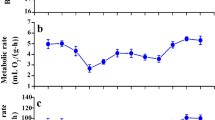
We examined development of endothermy in altricial Red-winged Blackbirds (Agelaius phoeniceus) by measuring oxygen consumption \((\dot{V}{\text{o}}_{2} )\), body temperature and ventilation at ambient temperatures from 35 to 15 °C. Mitochondrial respiration of permeabilized skeletal muscle was also measured from breast (pectoralis) and thigh (femorotibialis) muscles. Animals were studied from the first day of hatching through fledging (12 days post-hatch, dph). Nestling whole-body metabolic rate began to show an endothermic response to cold temperature midway between hatching and fledging. Nestlings less than 5 dph were unable to maintain elevated \(\dot{V}{\text{o}}_{2}\) and body temperature when exposed to gradually decreasing temperature, whereas 7 dph nestlings maintained \(\dot{V}{\text{o}}_{2}\) until ~25 °C, after which \(\dot{V}{\text{o}}_{2}\) decreased. From 10 dph to fledging, animals maintained elevated \(\dot{V}{\text{o}}_{2}\) and body temperature when exposed to gradual cooling; full endothermic capacity was achieved. Ventilation followed a similar developmental trend to that of \(\dot{V}{\text{o}}_{2}\), with increases in 10 dph fledglings occurring in tidal volume rather than ventilation frequency. LEAK respiration and oxidative phosphorylation (OXPHOS) through complex I of breast muscle mitochondria increased significantly after 3 dph. Expression of avUCP and PCG-1α mRNA increased significantly at 3 dph and remained elevated in both skeletal muscle types. Increased metabolic capacity at the cellular level occurred prior to that of the whole animal. This change in whole animal metabolic capacity increased steadily upon hatching as evidenced by the shift of metabolic rate from an ectothermic to endothermic phenotype and the increase of mitochondrial OXPHOS activity of the shivering muscles of this altricial avian species.





Similar content being viewed by others
References
Andreyev AY, Bondareva T, Dedukhova V, Mokhova E, Skulachev V, Tsofina L, Volkov N, Vygodina T (1989) The ATP/ADP antiporter is involved in the uncoupling effect of fatty acids. Anion carriers of mitochondrial membranes. Springer, Berlin, pp 159–168
Austin GT, Ricklefs RE (1977) Growth and development of the Rufous-winged Sparrow (Aimophila carpalis). Condor 79:37–50
Baarendse PJJ, Debonne M, Decuypere E, Kemp B, Van Den Brand H (2007) Ontogeny of avian thermoregulation from a neural point of view. Worlds Poult Sci J 63:267–276
Bech C, Østnes J (1999) Influence of body composition on the metabolic rate of nestling European shags (Phalacrocorax aristotelis). J Comp Physiol B 169:263–270
Brand M (2000) Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol 35:811–820
Brand MD, Esteves TC (2005) Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab 2:85–93
Cadenas S, Buckingham JA, St-Pierre J, Dickinson K, Jones RB, Brand MD (2000) AMP decreases the efficiency of skeletal-muscle mitochondria. Biochem J 351:307–311
Choi I, Ricklefs RE, Shea RE (1993) Skeletal muscle growth, enzyme activities, and the development of thermogenesis: a comparison between altricial and precocial birds. Physiol Zool 66:455–473
Collin A, Buyse J, van As P, Darras VM, Malheiros RD, Moraes VMB, Reyns GE, Taouis M, Decuypere E (2003) Cold-induced enhancement of avian uncoupling protein expression, heat production, and triiodothyronine concentrations in broiler chicks. Gen Comp Endocrinol 130:70–77
Dawson WR, Evans FC (1960) Relation of growth and development to temperature regulation in nestling vesper sparrows. Condor 62:329–340
Dawson WR, Whittow GC (2000) Regulation of body temperature. In: Whittow GC (ed) Sturkie’s Avian Physiology. Academic Press, New York, pp 343–390
Dégletagne D, Roussel D, Rouanet JL, Baudimont F, Moureaux E, Harvey S, Duchamp C, LeMaho Y, Raccurt M (2013) Growth prior to thermogenesis for a quick fledging of Adélie penguin chicks (Pygoscelis adeliae). PLOS One 8:e74154
Desvergne B, Michalik L, Wahli W (2004) Be fit or be sick: peroxisome proliferator-activated receptors are down the road. Mol Endocrinol 18:1321–1332
Dietz MW (1995) Development of metabolism and thermoregulation in galliforms. Effects of body mass, growth rate and functional maturity. Ph.D. thesis, Utrecht University, The Netherlands
Duchamp C, Barre H (1993) Skeletal muscle as the major site of nonshivering thermogenesis in cold-acclimated ducklings. Am J Physiol Regulatory Integrative Comp Physiol 265(5):R1076–R1083
Dunn EH (1976) Development of endothermy and existence energy expenditure of nestling double-crested cormorants. Condor 78:350–356
Dzialowski EM, Burggren WW, Komoro T, Tazawa H (2007) Development of endothermic metabolic response in embryos and hatchlings of the emu (Dromaius novaehollandiae). Respir Physiol Neurobiol 155:286–292
Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otín M, Pamplona R, Vidal-Puig AJ, Wang S, Roebuck SJ (2003) A signaling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J 22:4103–4110
Esteves TC, Brand MD (2005) The reactions catalyzed by the mitochondrial uncoupling proteins UCP2 and UCP3. BBA-Bioenergetics 1709:35–44
Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J (2001) Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J 15:2048–2050
Hirabayashi M, Ijiri D, Kamei Y, Tajima A, Kanai Y (2005) Transformation of skeletal muscle from fast- to slow-twitch during acquisition of cold tolerance in the chick. Endocrinol 146:399–405
Hohtola E (2004) Shivering thermogenesis in birds and mammals. In: Life in the cold: evolution, mechanisms, adaptation, and application. 12th International Hibernation Symposium, pp 241–252
Hohtola E, Visser H (1998) Development of locomotion and endothermy in altricial and precocial birds. In: Starck JM, Ricklefs RE (eds) Avian growth and development. Oxford University Press, Oxford, pp 157–173
Hood DA, Irrcher I, Ljubicic V, Joseph A (2006) Coordination of metabolic plasticity in skeletal muscle. J Exp Biol 209:2265–2275
Houten SM, Auwerx J (2004) PGC-1α: turbocharging mitochondria. Cell 119:5–7
Hulbert AJ, Else PL (2000) Mechanisms underlying the cost of living in animals. Annu Rev Physiol 62:207–235
Johnston DW (1971) The absence of brown adipose tissue in birds. Comp Biochem Physiol A 40:1107–1108
Kahl MP (1962) Bioenergetics of growth in nestling wood storks. Condor 64:169–183
Kuroda O, Matsunaga C, Whittow GC, Tazawa H (1990) Comparative metabolic responses to prolonged cooling in precocial duck (Anas domestica) and altricial pigeon (Columba domestica) embryos. Comp Biochem Physiol A 95:407–410
LeMoine CM, Lougheed SC, Moyes CD (2010) Modular evolution of PGC-1α in vertebrates. J Mol Evol 70:492–505
Lilja C (1983) A comparative study of postnatal growth and organ development in some species of birds. Growth 47:317–339
Marjoniemi K, Hohtola E (1999) Shivering thermogenesis in leg and breast muscles of galliform chicks and nestlings of the domestic pigeon. Physiol Biochem Zool 72:484–492
Matsunaga C, Mathiu P, Whittow G, Tazawa H (1989) Oxygen consumption of brown noddy (Anous stolidus) embryos in a quasiequilibrium state at lowered ambient temperatures. Comp Biochem Physiol A 93:707–710
McNabb FM, Stanton FW, Dicken SG (1984) Post-hatching thyroid development and body growth in precocial vs altricial birds. Comp Biochem Physiol A 18:629–635
Mezentseva NV, Kumaratilake JS, Newman SA (2008) The brown adipocyte differentiation pathway in birds: an evolutionary road not taken. BMC Biol 6:17
Mujahid A, Sato K, Akiba Y, Toyomizu M (2006) Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult Sci 85:1259–1265
Mujahid A, Akiba Y, Toyomizu M (2007) Acute heat stress induces oxidative stress and decreases adaptation in young white leghorn cockerels by downregulation of avian uncoupling protein. Poult Sci 86:364–371
Mujahid A, Akiba Y, Toyomizu M (2009) Olive oil-supplemented diet alleviates acute heat stress-induced mitochondrial ROS production in chicken skeletal muscle. Am J Physiol Regul Integr Comp Physiol 297:R690–R698
Olson JM (1992) Growth, the development of endothermy, and the allocation of energy in red-winged blackbirds (Agelaius phoeniceus) during the nestling period. Physioll Zool 65:124–152
Olson JM (1994) The ontogeny of shivering thermogenesis in the Red-winged Blackbird (Agelaius phoeniceus). J Exp Biol 191:59–88
Olson JM (2001) Ontogeny of catabolic and morphological properties of skeletal muscle of the red-winged blackbird (Agelaius phoeniceus). J Comp Physiol B 171:527–542
Olson JM, Dawson WR, Camilliere JJ (1988) Fat from Black-capped Chickadees: avian brown adipose tissue? Condor 90:529–537
Olson JM, McNabb FM, Jablonski MS, Ferris DV (1999) Thyroid development in relation to the development of endothermy in the Red-winged Blackbird (Agelaius phoeniceus). Gen Comp Endocrinol 116:204–212
Pesta D, Gnaiger E (2012) High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810:25–58
Price ER, Paladino FV, Strohl KP, Santidrián P, Klann K, Spotila JR (2007) Respiration in neonate sea turtles. Comp Biochem Physiol A 146:422–428
Raimbault S, Dridi S, Denjean F, Lachuer J, Couplan E, Bouillaud F, Bordas A, Duchamp C, Taouis M, Ricquier D (2001) An uncoupling protein homologue putatively involved in facultative muscle thermogenesis in birds. Biochem J 353:441–444
Rey B, Roussel D, Romestaing C, Belouze M, Rouanet JL, Desplanches D, Sibille B, Servais S, Duchamp C (2010) Up-regulation of avian uncoupling protein in cold-acclimated and hyperthyroid ducklings prevents reactive oxygen species production by skeletal muscle mitochondria. BMC Physiol 10:5
Rhen T, Metzger K, Schroeder A, Woodward R (2007) Expression of putative sex-determining genes during the thermosensitive period of gonad development in the snapping turtle, Chelydra serpentina. Sex Dev 1:255–270
Ricklefs RE (1983) Avian postnatal development. Avian Biol 7:1–83
Ricklefs RE, Williams JB (2003) Metabolic responses of shorebird chicks to cold stress: hysteresis of cooling and warming phases. J Exp Biol 206:2883–2893
Rolfe DFS, Brand MD (1997) The physiological significance of mitochondrial proton leak in animal cells and tissues. Biosci Rep 17:9–16
Saarela S, Keith JS, Hohtola E, Trayhurn P (1991) Is the “mammalian” brown fat-specific mitochondrial uncoupling protein present in adipose tissues of birds? Comp Biochem Physiol B 100:45–49
Sbong S, Dzialowski EM (2007) Respiratory and cardiovascular responses to acute hypoxia and hyperoxia in internally pipped chicken embryos. Comp Biochem Physiol A 148:761–768
Schaeffer PJ (1998) The development of the ventilatory response to cold in very young rats. Comp Biochem Physiol A 119:407–414
Sirsat TS and Dzialowski EM (2016) Ventilation changes associated with hatching and maturation of an endothermic phenotype in the Pekin duck, Anas platyrhynchos. Am J Physiol Regul Integr Comp Physiol. doi: 10.1152/ajpregu.00274.2015
Sirsat SKG, Sirsat TS, Faber A, Duquaine A, Winnick S, Sotherland PR, Dzialowski EM (2016a) Development of endothermy and concomitant increases in cardiac and skeletal muscle mitochondrial respiration in the precocial Pekin Duck (Anas platyrhynchos domestica). J Exp Biol. doi:10.1242/jeb.132282
Sirsat SKG, Sirsat TS, Price ER, Dzialowski EM (2016b) Post-hatching development of mitochondrial function, organ mass, and metabolic rate in two ectotherms, the American alligator (Alligator mississippiensis) and the common snapping turtle (Chelydra serpentina). Biol Open. doi:10.1242/bio.017160
Skulachev VP (1998) Uncoupling: new approaches to an old problem of bioenergetics. BBA-Bioenergetics 1363:100–124
Starck JM (1996) Intestinal growth in altricial European Starling (Sturnus vulgaris) and precocial Japanese Quail (Coturnix coturnix japonica). Cells Tissues Organs 156:289–306
Starck JM, Ricklefs RE (1998) Avian growth and development: evolution within the altricial-precocial spectrum. Oxford University Press, New York
Szdzuy K, Mortola JP (2007) Monitoring breathing in avian embryos and hatchlings by the barometric technique. Respir Physiol Neurobiol 159:241–244
Talbot DA, Hanuise N, Rey B, Rouanet JL, Duchamp C, Brand MD (2003) Superoxide activates a GDP-sensitive proton conductance in skeletal muscle mitochondria from king penguin (Aptenodytes patagonicus). Biochem Biophys Res Commun 312:983–988
Talbot DA, Duchamp C, Rey B, Hanuise N, Rouanet JL, Sibille B, Brand MD (2004) Uncoupling protein and ATP/ADP carrier increase mitochondrial proton conductance after cold adaptation of King Penguins. J Physiol 558:123–135
Tazawa H, Wakayama H, Turner JS, Paganelli CV (1988) Metabolic compensation for gradual cooling in developing chick embryos. Comp Biochem Physiol 89A:125–129
Ueda M, Watanabe K, Sato K, Akiba Y, Toyomizu M (2005) Possible role for avPGC-1α in the control of expression of fiber type, along with avUCP and avANT mRNAs in the skeletal muscles of cold-exposed chickens. FEBS Lett 579:11–17
Walczak R, Tontonoz P (2002) PPARadigms and PPARadoxes: expanding roles for PPARγ in the control of lipid metabolism. J Lip Res 43:177–186
Walter I, Seebacher F (2007) Molecular mechanisms underlying the development of endothermy in birds (Gallus gallus): a new role of PGC-1 alpha? Am J Physiol Regul Integr Comp Physiol 293:R2315–R2322
Walter I, Seebacher F (2009) Endothermy in birds: underlying molecular mechanisms. J Exp Biol 212:2328–2336
Whittow GC, Tazawa H (1991) The early development of thermoregulation in birds. Physiol Zool 64:1371–1390
Withers PC (2001) Design, calibration and calculation for flow-through respirometry systems. Aust J Zool 49:445–461
Acknowledgments
We thank two anonymous referees whose comments and suggestions greatly improved our manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
IOS 1146758 from the National Science Foundation to EMD.
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
Sirsat, S.K.G., Sirsat, T.S., Crossley, J.L. et al. The 12-day thermoregulatory metamorphosis of Red-winged Blackbirds (Agelaius phoeniceus). J Comp Physiol B 186, 651–663 (2016). https://doi.org/10.1007/s00360-016-0978-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-0978-0