Abstract
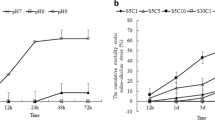
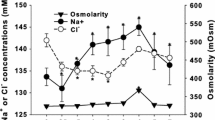
Naked carp (Gymnocypris przewalskii), endemic to the saline-alkaline Lake Qinghai, have the capacity to tolerate combined high salinity and alkalinity, but migrate to spawn in freshwater rivers each year. In this study, the full-length cDNA of the cytosolic carbonic anhydrase c isoform of G. przewalskii (GpCAc) was amplified and sequenced; mRNA levels and enzyme activity of GpCAc and blood chemistry were evaluated to understand the compensatory responses as the naked carp returned to the saline-alkaline lake after spawning. We found that GpCAc had a total length of 1400 bp and encodes a peptide of 260 amino acids. Comparison of the deduced amino acid sequences and phylogenetic analysis showed that GpCAc was a member of the cytosolic carbonic anhydrase II-like c family. Cytosolic-carbonic-anhydrase-c-specific primers were used to analyze the tissue distribution of GpCAc mRNA expression. Expression of GpCAc mRNA was found in brain, gill, liver, kidney, gut, and muscle tissues, but primarily in the gill and posterior kidney; however, none was evident in red blood cells. Transferring fish from river water to lake water resulted in a respiratory alkalosis, osmolality, and ion rise in the blood, as well as significant decreases in the expression and enzyme activity of GpCAc in both the gill and kidney within 96 h. These results indicate that GpCAc may play an important role in the acclimation to both high salinity and carbonate alkalinity. Specifically, G. przewalskii decreases cytosolic carbonic anhydrase c expression to compensate for a respiratory alkalosis and to aid in osmoregulation during the transition from river to saline-alkaline lake.






Similar content being viewed by others
References
Boutet I, Long Ky CL, Bonhomme F (2006) A transcriptomic approach of salinity response in the euryhaline teleost, Dicentrarchus labrax. Gene 379:40–50. doi:10.1016/j.gene.2006.04.011
Boyd CE (1992) Water quality and pond soil analyses for aquaculture. Alabama Agricultural Experiment Station, Auburn University, Auburn
Boyle D, Clifford AM, Orr E, Chamot D, Goss GG (2015) Mechanisms of Cl− uptake in rainbow trout: cloning and expression of slc26a6, a prospective Cl−/HCO3 − exchanger. Comp Biochem Physiol A Mol Integr Physiol 180:43–50. doi:10.1016/j.cbpa.2014.11.001
Cao YB, Chen XQ, Wang S, Chen XC, Wang YX, Chang JP, Du JZ (2009) Growth hormone and insulin-like growth factor of naked carp (Gymnocypris przewalskii) in Lake Qinghai: expression in different water environments. Gen Comp Endocrinol 161:400–406. doi:10.1016/j.ygcen.2009.02.005
Eladari D, Kumai Y (2015) Renal acid-base regulation: new insights from animal models. Pflugers Arch 467:1623–1641. doi:10.1007/s00424-014-1669-x
Esbaugh A, Tufts B (2006) The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Respir Physiol Neurobiol 154:185–198. doi:10.1016/j.resp.2006.03.007
Esbaugh AJ, Lund SG, Tufts BL (2004) Comparative physiology and molecular analysis of carbonic anhydrase from the red blood cells of teleost fish. J Comp Physiol [B] 174:429–438. doi:10.1007/s00360-004-0430-8
Esbaugh AJ, Perry SF, Bayaa M, Georgalis T, Nickerson J, Tufts BL, Gilmour KM (2005) Cytoplasmic carbonic anhydrase isozymes in rainbow trout Oncorhynchus mykiss: comparative physiology and molecular evolution. J Exp Biol 208:1951–1961. doi:10.1242/jeb.01551
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177. doi:10.1152/physrev.00050.2003
Evans DH, Claiborne JB, Currie S (2013) The Physiology of Fishes, 4th edn. CRC Press, Boca Raton
Fujikawa-Adachi K, Nishimori I, Taguchi T, Onishi S (1999) Human mitochondrial carbonic anhydrase VB. cDNA cloning, mRNA expression, subcellular localization, and mapping to chromosome x. J Biol Chem 274:21228–21233. doi:10.1074/jbc.274.30.21228
Geers C, Gros G (2000) Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev 80:681–715
Georgalis T, Gilmour K, Yorston J, Perry SF (2006a) Roles of cytosolic and membrane-bound carbonic anhydrase in renal control of acid-base balance in rainbow trout, Oncorhynchus mykiss. Am J Physiol Renal Physiol 291:F407–F421. doi:10.1152/ajprenal.00328.2005
Georgalis T, Perry S, Gilmour K (2006b) The role of branchial carbonic anhydrase in acid-base regulation in rainbow trout (Oncorhynchus mykiss). J Exp Biol 209:518–530. doi:10.1242/jeb.029181
Gilmour KM (2010) Perspectives on carbonic anhydrase. Comp Biochem Physiol A Mol Integr Physiol 157:193–197. doi:10.1016/j.cbpa.2010.06.161
Gilmour KM, Perry SF (2009) Carbonic anhydrase and acid-base regulation in fish. J Exp Biol 212:1647–1661. doi:10.1242/jeb.029181
Gilmour K, Perry S, Esbaugh A, Genz J, Taylor J, Grosell M (2012) Compensatory regulation of acid–base balance during salinity transfer in rainbow trout (Oncorhynchus mykiss). J Comp Physiol B 182:259–274. doi:10.1007/s00360-011-0617-8
Henry RP (1984) The role of carbonic anhydrase in blood ion and acid-base regulation. Am Zool 24:241–251. doi:10.1093/icb/24.1.241
Henry RP (1988) Multiple functions of carbonic anhydrase in the crustacean gill. J Exp Zool 248:19–24. doi:10.1002/jez.1402480104
Henry R (1991) Techniques for measuring carbonic anhydrase activity in vitro. In: Dodgson S, Tashian R, Gros G, Carter N (eds) The carbonic anhydrases: cellular pysiology and molecular genetics. Plenum, New York, pp 119–125. doi:10.1007/978-1-4899-0750-9_8
Henry RP, Swenson ER (2000) The distribution and physiological significance of carbonic anhydrase in vertebrate gas exchange organs. Respir Physiol 121:1–12. doi:10.1016/S0034-5687(00)00110-9
Henry R, Tufts B, Boutilier R (1993) The distribution of carbonic anhydrase type I and II isozymes in lamprey and trout: possible co-evolution with erythrocyte chloride/bicarbonate exchange. J Comp Physiol B 163:380–388. doi:10.1007/BF00265642
Hirata T et al (2003) Mechanism of acid adaptation of a fish living in a pH 3.5 lake. Am J Physiol Regul Integr Comp Physiol 284:R1199–R1212. doi:10.1152/ajpregu.00267.2002
Hwang P-P, Lee T-H, Lin L-Y (2011) Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol Regul Integr Comp Physiol 301:R28–R47. doi:10.1152/ajpregu.00047.2011
Johansen K, Maloiy GM, Lykkeboe G (1975) A fish in extreme alkalinity. Respir Physiol 24:159–162. doi:10.1016/0034-5687(75)90110-3
Larsen EH, Deaton LE, Onken H, O’Donnell M, Grosell M, Dantzler WH, Weihrauch D (2014) Osmoregulation and excretion. Comprehensive. Physiology 4:405–573. doi:10.1002/cphy.c130004
Lehtonen J et al (2004) Characterization of CA XIII, a novel member of the carbonic anhydrase isozyme family. J Biol Chem 279:2719–2727. doi:10.1074/jbc.M308984200
Lin TY, Liao BK, Horng JL, Yan JJ, Hsiao CD, Hwang PP (2008) Carbonic anhydrase 2-like a and 15a are involved in acid-base regulation and Na+ uptake in zebrafish H+-ATPase-rich cells. Am J Physiol Cell Physiol 294:C1250–C1260. doi:10.1152/ajpcell.00021.2008
Lin CC, Lin LY, Hsu HH, Thermes V, Prunet P, Horng JL, Hwang PP (2012) Acid secretion by mitochondrion-rich cells of medaka (Oryzias latipes) acclimated to acidic freshwater. Am J Physiol Regul Integr Comp Physiol 302:R283–R291. doi:10.1152/ajpregu.00483.2011
Lindskog S (1997) Structure and mechanism of carbonic anhydrase. Pharmacol Ther 74:1–20. doi:10.1016/S0163-7258(96)00198-2
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Marshall W, Grosell M (2006) Ion transport and osmoregulation in fish. In: Dvans D (ed) The physiology of fishes. CRC Press, Boca Raton
Perry SF, Fryer JN (1997) Proton pumps in the fish gill and kidney. Fish Physiol Biochem 17:363–369. doi:10.1023/A:1007746217349
Perry S, Gilmour K (2006) Acid–base balance and CO2 excretion in fish: unanswered questions and emerging models. Respir Physiol Neurobiol 154:199–215. doi:10.1016/j.resp.2006.04.010
Perry S, Shahsavarani A, Georgalis T, Bayaa M, Furimsky M, Thomas S (2003) Channels, pumps, and exchangers in the gill and kidney of freshwater fishes: their role in ionic and acid-base regulation. J Exp Zool Part A Comp Exp Biol 300:53–62. doi:10.1002/jez.a.10309
Piermarini PM, Verlander JW, Royaux IE, Evans DH (2002) Pendrin immunoreactivity in the gill epithelium of a euryhaline elasmobranch. Am J Physiol Regul Integr Comp Physiol 283:R983–R992. doi:10.1152/ajpregu.00178.2002
Pongsomboon S, Udomlertpreecha S, Amparyup P, Wuthisuthimethavee S, Tassanakajon A (2009) Gene expression and activity of carbonic anhydrase in salinity stressed Penaeus monodon. Comp Biochem Physiol A Mol Integr Physiol 152:225–233. doi:10.1016/j.cbpa.2008.10.001
Randall DJ, Tsui TK (2006) Tribute to R. G. Boutilier: acid-base transfer across fish gills. J Exp Biol 209:1179–1184. doi:10.1242/jeb.02100
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. In: Misener S, Krawetz SA (eds) Methods in molecular biology. Bioinformatics methods and protocols, vol 132. Humana Press, Totowa, pp 365–386. doi:10.1385/1-59259-192-2:365
Rychlik W (2007) OLIGO 7 primer analysis software. In: Yuryev A (ed) Methods Mol Biol. PCR primer design, vol 402. Humana Press, Totowa, pp 35–60. doi:10.1007/978-1-59745-528-2_2
Sachs AB (1993) Messenger RNA degradation in eukaryotes. Cell 74:413–421. doi:10.1016/0092-8674(93)80043-E
Santovito G, Marino SM, Sattin G, Cappellini R, Bubacco L, Beltramini M (2012) Cloning and characterization of cytoplasmic carbonic anhydrase from gills of four Antarctic fish: insights into the evolution of fish carbonic anhydrase and cold adaptation. Polar Biol 35:1587–1600. doi:10.1007/s00300-012-1200-9
Scott GR, Claiborne JB, Edwards SL, Schulte PM, Wood CM (2005) Gene expression after freshwater transfer in gills and opercular epithelia of killifish: insight into divergent mechanisms of ion transport. J Exp Biol 208:2719–2729. doi:10.1242/jeb.01688
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599. doi:10.1093/molbev/msm092
Tresguerres M, Parks SK, Wood CM, Goss GG (2007) V-H+ -ATPase translocation during blood alkalosis in dogfish gills: interaction with carbonic anhydrase and involvement in the postfeeding alkaline tide. Am J Physiol Regul Integr Comp Physiol 292:R2012–R2019. doi:10.1152/ajpregu.00814.2006
Tresguerres M, Barott KL, Barron ME, Roa JN (2014) Established and potential physiological roles of bicarbonate-sensing soluble adenylyl cyclase (sAC) in aquatic animals. J Exp Biol 217:663–672. doi:10.1242/jeb.086157
Walker GA (2010) Common statistical methods for clinical research with SAS examples. SAS Institute, Cary
Walker K, Dunn I, Edwards D, Petr T, Yang H (1995) A fishery in a changing lake environment: the naked carp Gymnocypris przewalskii (Kessler) (Cyprinidae: schizothoracinae) in Qinghai Hu, China. Int J Salt Lake Res 4:169–222. doi:10.1007/BF02001491
Wang S, Xie Y (2009) China species red list. Higher education press, Beijing
Wang YS et al (2003) Unusual physiology of scale-less carp, Gymnocypris przewalskii, in Lake Qinghai: a high altitude alkaline saline lake. Comp Biochem Physiol A Mol Integr Physiol 134:409–421. doi:10.1016/S1095-6433(02)00317-3
Wilkie MP, Wood CM (1996) The adaptations of fish to extremely alkaline environments. Comp Biochem Physiol B Biochem Mol Biol 113:665–673. doi:10.1016/0305-0491(95)02092-6
Wilkie MP, Wright PA, Iwama GK, Wood CM (1994) The physiological adaptations of the Lahontan cutthroat trout (Oncorhynchus clarki henshawi) following transfer from well water to the highly alkaline waters of Pyramid Lake, Nevada (pH 9.4). Physiol Zool. doi:10.2307/30163853
Wilson RW, Millero FJ, Taylor JR, Walsh PJ, Christensen V, Jennings S, Grosell M (2009) Contribution of fish to the marine inorganic carbon cycle. Science 323:359–362. doi:10.1126/science.1157972
Wood CM et al (2007) Przewalski’s naked carp (Gymnocypris przewalskii): an endangered species taking a metabolic holiday in Lake Qinghai, China. Physiol Biochem Zool 80:59–77. doi:10.1086/509212
Xiong F, Chen D, Duan X (2010) Threatened fishes of the world: Gymnocypris przewalskii (Kessler, 1876) (Cyprinidae: Schizothoracinae). Environ Biol Fishes 87:351–352. doi:10.1007/s10641-010-9609-x
Yang L, Chen J, Chang CC, Yang X-Y, Wang Z-Z, Chang T-Y, Li B-L (2004) A stable upstream stem-loop structure enhances selection of the first 5′-ORF-AUG as a main start codon for translation initiation of human ACAT1 mRNA. Acta Biochim Biophys Sin 36:259–268. doi:10.1093/abbs/36.4.259
Acknowledgments
This study was supported by Special Scientific Research Funds for Central Non-profit Institutes (East China Sea Fisheries Research Institute) (No. 2012M05) and National Natural Science Foundation of China (No. 31440088). We would like to thank Dr. Edward Mager and Dr. Martin Grosell for their insightful comments on an earlier version of this manuscript. We would also like to thank Dr. Katie Gilmour for providing perfusion methods.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by H.V. Carey.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yao, Z., Guo, W., Lai, Q. et al. Gymnocypris przewalskii decreases cytosolic carbonic anhydrase expression to compensate for respiratory alkalosis and osmoregulation in the saline-alkaline lake Qinghai. J Comp Physiol B 186, 83–95 (2016). https://doi.org/10.1007/s00360-015-0939-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-015-0939-z