Abstract
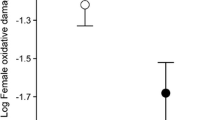
The study of oxidative stress is a potential tool for studying the functional interactions among life history traits, sexual traits and physiological status in animals. In this study, we investigated relationships between measures of plasma oxidative status and male sexual traits, female reproductive investment and three other life history traits, in a wild population of collared flycatchers (Ficedula albicollis). Flycatcher males with a larger white forehead patch had higher level of plasma antioxidant capacity. For females, clutch size was not associated with plasma oxidative status, but egg size was positively correlated with antioxidant capacity. The relationship between age and levels of plasma oxidative damage remains controversial in this species: young female flycatchers showed higher levels of hydroperoxides compared to antioxidants, whereas age did not predict oxidative status of males. Males had higher levels of oxidative damage than females, although the concentration of antioxidant compounds was similar between the sexes. Females that mated with more ornamented males had higher plasma antioxidant capacity. Our results suggest that, for males and females, greater investment in sexual signal and reproduction, respectively, does not reduce the capacity for self-maintenance or avoidance of oxidative stress. Finally, our data support indirectly the occurrence of assortative mating in our species, since females with higher plasma antioxidant capacity mated with more ornamented males.



Similar content being viewed by others
References
Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G (2004) Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol Lett 7:363–368
Alonso-Alvarez C, Bertrand S, Faivre B, Chastel O, Sorci G (2007) Testosterone and oxidative stress: the oxidation handicap hypothesis. Proc Roy Soc Lond B 274:819–825
Alonso-Alvarez C, Pérez-Rodriguez L, Garcia JT, Viñuela J, Mateo R (2010) Age and breeding effort as sources of individual variability in oxidative stress markers in a bird species. Physiol Biochem Zool 83:110–118
Andersson M, Iwasa Y (1996) Sexual selection. Trends Ecol Evol 11:53–58
Badyaev AV (2004) Developmental perspective on the evolution of sexual ornaments. Evol Ecol Res 6:975–991
Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78:547–581
Bertrand S, Alonso-Alvarez C, Devevey G, Faivre B, Prost J, Sorci G (2006) Carotenoids modulate the trade-off between egg production and resistance to oxidative stress in zebra finches. Oecologia 147:576–584
Bize P, Devevey G, Monaghan P, Doligez B, Christe P (2008) Fecundity and survival in relation to resistance to oxidative stress in a free-living bird. Ecology 89:2584–2593
Chastel O, Lacroix A, Kersten M (2003) Pre-breeding energy requirements: thyroid hormone, metabolism and the timing of reproduction in house sparrows Passer domesticus. J Avian Biol 34:298–306
Costantini D (2008) Oxidative stress in ecology and evolution: lessons from avian studies. Ecol Lett 11:1238–1251
Costantini D (2010) Effects of diet quality on serum oxidative status and body mass in male and female pigeons during reproduction. Comp Biochem Physiol Part A 156:294–299
Costantini D, Dell’Omo G (2006) Effects of T-cell-mediated immune response on avian oxidative stress. Comp Biochem Physiol Part A 145:137–142
Costantini D, Verhulst S (2009) Does high antioxidant capacity indicate low oxidative stress? Funct Ecol 23:506–509
Costantini D, Casagrande S, De Filippis S, Brambilla G, Fanfani A, Tagliavini J, Dell’Omo G (2006) Correlates of oxidative stress in wild kestrel nestlings (Falco tinnunculus). J Comp Physiol B 176:329–337
Costantini D, Coluzza C, Fanfani A, Dell’Omo G (2007) Effects of carotenoid supplementation on colour expression, oxidative stress and body mass in rehabilitated captive adult kestrels (Falco tinnunculus). J Comp Physiol B 177:723–731
Costantini D, Dell’Ariccia G, Lipp HP (2008) Long flights and age affect oxidative status of homing pigeons (Columba livia). J Exp Biol 211:377–381
Devevey G, Bruyndonckx N, von Houwald F, Studer-Thiersch A, Christie P (2010) Age-specific variation of resistance to oxidative stress in the greater flamingo (Phoenicopterus ruber roseus). J Ornithol 151:251–254
Dowling DK, Simmons LW (2009) Reactive oxygen species as universal constraints in life-history evolution. Proc R Soc Lond B 276:1737–1745
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Garamszegi LZ, Møller AP, Török J, Michl G, Péczely P, Richard M (2004) Immune challenge mediates vocal communication in a passerine bird: an experiment. Behav Ecol 15:148–157
Grafen A (1990) Biological signals as handicaps. J Theor Biol 144:517–546
Gustafsson L, Qvarnström A, Sheldon BC (1995) Trade-offs between life-history traits and a secondary sexual character in male collared flycatchers. Nature 375:311–313
Halliwell BH, Gutteridge JMC (2007) Free radicals in biology and medicine. Oxford University Press, Oxford
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds—a role for parasites. Science 218:384–387
Hargitai R, Herényi M, Török J (2008) Eggshell coloration in relation to male ornamentation, female condition and egg quality in the collared flycatcher Ficedula albicollis. J Avian Biol 39:413–422
Hartley RC, Kennedy MW (2004) Are carotenoids a red herring in sexual display? Trends Ecol Evol 19:353–354
Hegyi G, Török J, Tóth L (2002) Qualitative population divergence in proximate determination of a sexually selected trait in the collared flycatcher. J Evol Biol 15:710–719
Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA (2007) Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 87:1175–1213
Ilmonen P, Taarna T, Hasselquist D (2000) Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proc Roy Soc Lond B 267:665–670
Johnston SL, Grune T, Bell LM, Murray SJ, Souter DM, Erwin SS, Yearsley JM, Gordon IJ, Illius AW, Kyriazakis I, Speakman JR (2006) Having it all: historical energy intakes do not generate the anticipated trade-offs in fecundity. Proc Roy Soc Lond B 273:1369–1374
Kokko H (2001) Fisherian and “good genes” benefits of mate choice: how (not) to distinguish between them. Ecol Lett 4:322–326
Kotiaho JS (2001) Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol Rev 76:365–376
Lozano GA (1994) Carotenoids, parasites, and sexual selection. Oikos 70:309–311
Michl G, Török J, Griffith SC, Sheldon BC (2002) Experimental analysis of sperm competition mechanisms in a wild bird population. Proc Natl Acad Sci USA 99:5466–5470
Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurement and interpretation. Ecol Lett 12:75–92
Mougeot F, Irvine JR, Seivwright L, Redpath SM, Piertney S (2004) Testosterone, immunocompetence, and honest sexual signaling in male red grouse. Behav Ecol 15:930–937
Nilsson JA, Raberg L (2001) The resting metabolic cost of egg laying and nestling feeding in great tits. Oecologia 128:187–192
Pamplona R, Barja G (2006) Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim Biophys Acta Bioenerg 1757:496–508
Pärt T, Qvarnström A (1997) Badge size in collared flycatchers predicts outcome of male competition over territories. Anim Behav 54:893–899
Partridge L, Gems D, Withers DJ (2005) Sex and death: what is the connection? Cell 120:461–472
Pérez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A (2009) Is the oxidative stress theory of aging dead? Biochim Biophys Acta 1790:1005–1014
Qvarnström A, Pärt T, Sheldon BC (2000) Adaptive plasticity in mate preference linked to differences in reproductive effort. Nature 405:344–347
Qvarnström A, Brommer JE, Gustafsson L (2006) Testing the genetics underlying the co-evolution of mate choice and ornament in the wild. Nature 441:84–86
Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. Trends Ecol Evol 17:462–468
Saetre GP, Moum T, Bures S, Kral M, Adamjan M, Moreno J (1997) A sexually selected character displacement in flycatchers reinforces premating isolation. Nature 387:589–592
Safran RJ, Adelman JS, McGraw KJ, Hau M (2008) Sexual signal exaggeration affects physiological state in male barn swallows. Curr Biol 18:R461–R462
Salmon AB, Richardson A, Pérez VI (2010) Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Rad Biol Med 48:642–655
Sanz A, Pamplona R, Barja G (2006) Is the mitochondrial free radical theory of aging intact? Antiox Redox Signal 8:582–599
Selman C, McLaren JS, Collins AR, Duthie GG, Speakman JR (2008) The impact of experimentally elevated energy expenditure on oxidative stress and lifespan in the short-tailed field vole Microtus agrestis. Proc Roy Soc Lond B 275:1907–1916
Sheldon BC, Ellegren H (1999) Sexual selection resulting from extrapair paternity in collared flycatchers. Anim Behav 57:285–298
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295
Speakman JR (2005) Body size, energy metabolism and lifespan. J Exp Biol 208:1717–1730
Svensson L (2002) Identification guide to European passerines. Svensson, Stockholm
Vassalle C (2008) An easy and reliable automated method to estimate oxidative stress in the clinical setting. In: Armstrong D (ed) Methods in Molecular Biology vol. 47: Advanced protocols in oxidative stress I. Humana Press, New York
von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H (1999) Good genes, oxidative stress and condition-dependent sexual signals. Proc Roy Soc Lond B 266:1–12
Welcker J, Moe B, Bech C, Fyhn M, Schultner J, Speakman JR, Gabrielsen GW (2010) Evidence for an intrinsic energetic ceiling in free-ranging kittiwakes Rissa tridactyla. J Anim Ecol 79:205–213
Wiersma P, Selman C, Speakman JR, Verhulst S (2004) Birds sacrifice oxidative protection for reproduction. Proc Roy Soc Lond B 271:S360–S363
Acknowledgments
We thank Mia Hoogenboom, Celeste R. West-Pongrácz and Péter Pongrácz for their comments on previous versions of the manuscript and for the linguistic revision. We are grateful to the members of the Behavioural Ecology Group for their assistance during the fieldwork. This study was supported by the Hungarian Scientific Research Fund (OTKA, grants no. K75618), the Eötvös Loránd University and Pilis Park Forestry. DC was supported by a postdoctoral NERC research fellowship (NE/G013888/1) during manuscript preparation. DC thanks the International Observatory for Oxidative Stress (Salerno, Italy) for advice and support; Gianfranco Brambilla and Edoardo Vignolo for technical and logistical support at the ISS, Rome.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Markó, G., Costantini, D., Michl, G. et al. Oxidative damage and plasma antioxidant capacity in relation to body size, age, male sexual traits and female reproductive performance in the collared flycatcher (Ficedula albicollis). J Comp Physiol B 181, 73–81 (2011). https://doi.org/10.1007/s00360-010-0502-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-010-0502-x