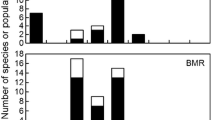
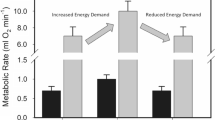
Abstract
Comparative analyses of avian energetics often involve the implicit assumption that basal metabolic rate (BMR) is a fixed, taxon-specific trait. However, in most species that have been investigated, BMR exhibits phenotypic flexibility and can be reversibly adjusted over short time scales. Many non-migrants adjust BMR seasonally, with the winter BMR usually higher than the summer BMR. The data that are currently available do not, however, support the idea that the magnitude and direction of these adjustments varies consistently with body mass. Long-distance migrants often exhibit large intra-annual changes in BMR, reflecting the physiological adjustments associated with different stages of their migratory cycles. Phenotypic flexibility in BMR also represents an important component of short-term thermal acclimation under laboratory conditions, with captive birds increasing BMR when acclimated to low air temperatures and vice versa. The emerging view of avian BMR is of a highly flexible physiological trait that is continually adjusted in response to environmental factors such as temperature. The within-individual variation observed in avian BMR demands a critical re-examination of approaches used for comparisons across taxa. Several key questions concerning the shapes and other properties of avian BMR reaction norms urgently need to be addressed, and hypotheses concerning metabolic adaptation should explicitly account for phenotypic flexibility.




Similar content being viewed by others
Abbreviations
- BMR:
-
Basal metabolic rate
- T a :
-
Air temperature
- M b :
-
Body mass
- M sum :
-
Summit metabolism
- FMR:
-
Field metabolic rate
- MMR:
-
Maximal metabolic rate
- T CL :
-
Temperature at cold limit
References
Adolph SC, Hardin JS (2007) Estimating phenotypic correlations: correcting for bias due to intraindividual variability. Funct Ecol 21:178–184
Afik D, Caviedes-Vidal E, Martinez del Rio C, Karasov WH (1995) Dietary modulation of intestinal hydrolytic enzymes in yellow-rumped warblers. Am J Physiol 269:R413–R420
Arens JR, Cooper SJ (2005) Metabolic and ventilatory acclimatization to cold stress in house sparrows (Passer domesticus). Physiol Biochem Zool 78:579–589
Battley PF, Dekinga A, Dietz MW, Piersma T, Tang S, Hulsman K (2001) Basal metabolic rate declines during long-distance migratory flight in great knots. Condor 103:838–845
Battley PF, Piersma T, Dietz MW, Tang S, Dekinga A, Hulsman K (2000) Empirical evidence for differential organ reductions during trans-oceanic bird flight. Proc R Soc Lond B 267:191–195
Bech C, Langseth I, Gabrielsen GW (1999) Repeatability of basal metabolism in breeding female kittiwakes Rissa tridactyla. Proc R Soc Lond B 266:2161–2167
Benedict FG, Fox EL (1927) The gaseous metabolism of large birds under aviary life. Proc Am Philos Soc 66:511–534
Bennett PM, Harvey PH (1987) Active and resting metabolism in birds: allometry, phylogeny and ecology. J Zool Lond 213:327–363
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745
Brody S, Proctor RC (1932) Growth and development, with special reference to domestic animals. XXIII. Relation between basal metabolism and mature body weight in different species of mammals and birds. Mo Univ Agric Exp Stn Res Bull 166:89–101
Broggi J, Hohtola E, Koivula K, Orell M, Thomson RL, Nilsson J-A (2007) Sources of variation in winter basal metabolic rate in the great tit. Funct Ecol 21:528–533
Carleton SA, Martinez del Rio C (2005) The effect of cold-induced increased metabolic rate on the rate of 13C and 15N incorporation in house sparrows (Passer domesticus). Oecologia 144:226–232
Chappell MA, Bech C, Buttemer WA (1999) The relationship of central and peripheral organ masses to aerobic performance variation in house sparrows. J Exp Biol 202:2269–2279
Chetty K (2006) Phenotypic flexibility in the basal metabolic rate of laughing doves (Streptopelia senegalensis) in response to short-term thermal acclimation. MSc. thesis, University of the Witwatersrand, Johannesburg
Chown SL, Terblanche JS (2007) Physiological diversity in insects: ecological and evolutionary contexts. Adv Insect Physiol 33:50–152
Cooper SJ (2000) Seasonal energetics of mountain chickadees and juniper titmice. Condor 102:635–644
Cooper SJ, Swanson DL (1994) Seasonal acclimatization of thermoregulation in the black-capped chickadee. Condor 96:638–646
Cossins AR, Bowler K (1987) Temperature biology of animals. Chapman and Hall, London
Daan S, Masman D, Groenewold A (1990) Avian basal metabolic rates: their association with body compostition and energy expenditure in nature. Am J Physiol 259:R333–R340
Davydov AF (1972) Seasonal variations in the energy metabolism and thermoregulation at rest in the black-headed gull. Sov J Ecol 2:436–439
Dawson WR (2003) Plasticity in avian responses to thermal challenges— an essay in honor of Jacob Marder. Isr J Zool 49:95–109
Dawson WR, Buttemer WA, Carey C (1985) A reexamination of the metabolic response of house finches to temperature. Condor 87:424–427
Dawson WR, Carey C (1976) Seasonal acclimation to temperature in Cardueline finches. J Comp Physiol 112:317–333
Dawson WR, Whittow GC (2000) Regulation of body temperature. In: Sturkie PD (ed) Avian physiology. Academic, New York, pp 343–390
Dietz MW, Piersma T, Dekinga A (1999) Body-building without power training: endogenously regulated pectoral muscle hypertrophy in confined shorebirds. J Exp Biol 202:2831–2837
Ellis HI (1984) Energetics of free-ranging seabirds. In: Whittow GC, Rahn H (eds) Seabird energetics. Plenum, New York
Freckleton RP, Harvey PH, Pagel M (2002) Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160:712–726
Garland T, Dickerman AW, Janis CM, Jones JA (1993) Phylogenetic analysis of covariance by computer simulation. System Biol 42:265–292
Garland T, Harvey PH, Ives AR (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. System Biol 41:18–32
Garland T, Ives AR (2000) Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative methods. Am Nat 155:346–364
Gelineo S (1964) Organ systems in adaptation: the temperature regulating system. In: Dill DB (ed) Handbook of physiology, Section 4, Adaptation to the environment. American Physiological Society, Washington DC
Gelineo S (1969) Heat production in birds in summer and winter. Srpska Akad. Nauka I Umetnosti Belgrad, Bull Classe Sci Math Sci Nat 12:99–105
Giaja J, Males B (1928) Sur la valeur du métabolisme de base de quelques animaux en fonction de leur surface. Annales de Physiologie et de Physicochimie Biologique 4:875–904
Hammond KA, Chappell MA, Cardullo RA, Lin R-I, Johnsen TS (2000) The mechanistic basis of aerobic performance variation in red junglefowl. J Exp Biol 203:2053–2064
Hart JS (1962) Seasonal acclimatization in four species of small wild birds. Physiol Zool 35:224–236
Haugen MJ, Tieleman BI, Williams JB (2003) Phenotypic flexibility in cutaneous water loss and lipids of the stratum corneum. J Exp Biol 206:3581–3588
Hayworth AM, Weathers WW (1984) Temperature regulation and climatic adaptation in black-billed and yellow-billed magpies. Condor 86:19–26
Hissa R, Palonkangas R (1970) Thermoregulation in the titmouse (Parus major L.). Comp Biochem Physiol 33:941–953
Hoffman TCM, Walsberg GE (1999) Inhibiting ventilatory evaporation produces an adaptive increase in cutaneous evaporation in mourning doves Zenaida macroura. J Exp Biol 202:3021–3028
Irving L, Krog H, Monson M (1955) The metabolism of some Alaskan animals in winter and summer. Physiol Zool 28:173–185
Ives AR, Midford PE, Garland T (2007) Within-species variation and measurement error in phylogenetic comparative methods. System Biol 56:252–270
Karasov WH (1996) Digestive plasticity in avian energetics and feeding ecology. In: Carey C (ed) Avian energetics and nutritional ecology. Chapman and Hall, New York, pp 61–84
Karasov WH, Pinshow B (1998) Changes in lean mass and in organs of nutrient assimilation in a long-distance passerine migrant at a spring stopover site. Physiol Biochem Zool 71:435–448
Kersten M, Bruinzeel LW, Wiersma P, Piersma T (1998) Reduced basal metabolic rate of migratory waders wintering in coastal Africa. Ardea 86:71–80
Klaassen M, Biebach H (1994) Energetics of fattening and starvation in the long-distance migratory garden warbler, Sylvia borin, during the migratory phase. J Comp Physiol B 164:362–371
Klaassen M, Oltrogge M, Trost L (2004) Basal metabolic rate, food intake, and body mass in cold- and warm-acclimated garden warblers. Comp Biochem Physiol A 137:639–647
Kvist A, Lindström Å (2001) Basal metabolic rate in migratory waders: intra-individual, intraspecific, interspecific and seasonal variation. Funct Ecol 15:465–473
Lasiewski RC, Dawson WR (1967) A re-examination of the relation between standard metabolic rate and body weight in birds. Condor 69:13–23
Levey DJ, Place AR, Rey PJ, Martinez del Rio C (1999) An experimental test of dietary enzyme modulation in pine warblers Dendroica pinus . Physiol Biochem Zool 72:576–587
Liknes ET, Scott SM, Swanson DL (2002) Seasonal acclimatization in the American goldfinch revisited: to what extent do metabolic rates vary seasonally? Condor 104:548–557
Liknes ET, Swanson DL (1996) Seasonal variation in cold tolerance, basal metabolic rate, and maximal capacity for thermogenesis in white-breasted nuthatches Sitta carolinensis and downy woodpeckers Picoides pubescens, two unrelated arboreal temperate residents. J Avian Biol 27:279–288
Lindström Å (1997) Basal metabolic rates of migrating waders in the Eurasian Arctic. J Avian Biol 28:87–92
Lindström Å, Klaassen M (2003) High basal metabolic rates of shorebirds while in the Arctic: a circumpolar view. Condor 105:420–427
Lindström Å, Klaassen M, Kvist A (1999) Variation in energy intake and basal metabolic rate of a bird migrating in a wind tunnel. Funct Ecol 13:352–359
Maddocks TA, Geiser F (2000) Seasonal variations in thermal energetics of Australian silvereyes (Zosterops lateralis). J Zool Lond 252:327–333
Marder J, Arieli U (1988) Heat balance of acclimated pigeons Columba livia exposed to temperatures of up to 60°C T a. Comp Biochem Physiol 91A:165–170
Martinez del Rio C, Brugger KW, Rios JL, Vergara ME, Witmer MC (1995) An experimental and comparative study of dietary modulation of intestinal enzymes in the European starling. Physiol Zool 68:490–511
Martins EP, Hansen TF (1997) Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am Nat 149:646–667
McKechnie AE, Chetty K, Lovegrove BG (2007) Phenotypic flexibility in basal metabolic rate in laughing doves: responses to short-term thermal acclimation. J Exp Biol 210:97–106
McKechnie AE, Freckleton RP, Jetz W (2006) Phenotypic plasticity in the scaling of avian basal metabolic rate. Proc R Soc Lond B 273:931–937
McKechnie AE, Lovegrove BG (2001) Thermoregulation and the energetic significance of clustering behavior in the white-backed mousebird (Colius colius). Physiol Biochem Zool 74:238–249
McKechnie AE, Lovegrove BG (2003) Facultative hypothermic responses in an Afrotropical arid-zone passerine, the red-headed finch (Amadina erythrocephala). J Comp Physiol B 173:339–346
McKechnie AE, Wolf BO (2004a) The allometry of avian basal metabolic rate: good predictions need good data. Physiol Biochem Zool 77:502–521
McKechnie AE, Wolf BO (2004b) Partitioning of evaporative water loss in white-winged doves: plasticity in response to short-term thermal acclimation. J Exp Biol 207:203–210
McNab BK (1988) Food habits and the basal rate of metabolism in birds. Oecologia 77:343–349
McNab BK (1997) On the utility of uniformity in the definition of basal rates of metabolism. Physiol Zool 70:718–720
McNab BK (2001) Energetics of toucans, barbets and a hornbill: implications for avian frugivory. Auk 118:916–933
McNab BK (2003) Ecology shapes bird bioenergetics. Nature 426:620–621
McNab BK (2005) Food habits and the evolution of energetics in birds of paradise (Paradisaeidae). J Compar Physiol B 175:117–132
Merola-Zwartjes M, Ligon JD (2000) Ecological energetics of the Puerto Rican tody: heterothermy, torpor and intra-island variation. Ecology 81:990–1002
Nagy KA (1987) Field metabolic rate and food requirement scaling in mammals and birds. Ecol Monogr 57:111–128
O’Conner TP (1995) Metabolic characteristics and body composition in house finches: effects of seasonal acclimatization. J Compar Physiol B 165:298–305
Pagel M (1994) Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc R Soc Lond B 255:37–45
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884
Piersma T (2002) Energetic bottlenecks and other design constraints in avian annual cycles. Integr Compar Biol 42:51–67
Piersma T, Cadée N, Daan S (1995) Seasonality in basal metabolic rate and thermal conductance in a long distance migrant shorebird, the knot (Calidris canutus). J Compar Physiol 165:37–45
Piersma T, Drent J (2003) Phenotypic flexibility and the evolution of organismal design. Trends Ecol Evol 18:228–233
Piersma T, Gessaman JA, Dekinga A, Visser GH (2004) Gizzard and other lean mass components increase, yet basal metabolic rates decrease, when red knots Calidris canutus are shifted from soft to hard-shelled food. J Avian Biol 35:99–104
Piersma T, Lindström A (1997) Rapid reversible changes in organ size as a component of adaptive behaviour. Trends Ecol Evol 12:134–138
Pohl H (1971) Seasonal variation in metabolic functions of bramblings. Ibis 113:185–193
Pohl H, West GC (1973) Daily and seasonal variation in metabolic response to cold during rest and exercise in the common redpoll. Comp Biochem Physiol 45A:851–867
Precht H (1973) Limiting temperatures of life functions. In: Precht H, Christophersen J, Hensel H, Larcher W (eds) Temperature and life. Spinger, Berlin, pp 400–440
Prosser CL (1973) Comparative animal physiology. Saunders, Philadelphia
Reynolds PS, Lee RM (1996) Phylogenetic analysis of avian energetics: passerines and non-passerines do not differ. Am Nat 147:735–759
Rezende EL, Swanson DL, Novoa FF, Bozinovic F (2002) Passerines versus nonpasserines: so far, no statistical differences in the scaling of avian energetics. J Exp Biol 205:101–107
Ricklefs RE, Konarzewski M, Daan S (1996) The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. Am Nat 147:1047–1071
Riddle O, Smith GC, Benedict FG (1934) Seasonal and temperature factors and their determination in pigeons of percentage metabolism change per degree of temperature change. Am J Physiol 107
Rising JD, Hudson JW (1974) Seasonal variation in the metabolism and thyroid activity of the black-capped chickadee (Parus atricapillus). Condor 76:198–203
Rønning B, Moe B, Bech C (2005) Long-term repeatability makes basal metabolic rate a likely heritable trait in the zebra finch Taeniopygia guttata. J Exp Biol 208:4663–4669
Saarela S, Hohtola E (2003) Seasonal thermal acclimatization in sedentary and active pigeons. Isr J Zool 49:185–193
Saarela S, Klapper B, Heldmaier G (1988) Thermogenic capacity of greenfinches and siskins in winter and summer. In: Bech C, Reinertsen RE (eds) Physiology of cold adaptation in birds. Plenum, New York, pp 115–122
Schleucher E, Withers PC (2001) Re-evaluation of the allometry of wet thermal conductance for birds. Comp Biochem Physiol A 129:821–827
Schlichting CD, Pigliucci M (1998) Phenotypic evolution: a reaction norm perspective. Sinauer Associates, Sunderland
Sharbaugh SM (2001) Seasonal acclimatization to extreme climatic conditions by black-capped chickadees (Poecile atricapilla) in interior Alaska (64°N). Physiol Biochem Zool 74:568–575
Southwick EE (1980) Seasonal thermoregulatory adjustments in white-crowned sparrows. Auk 97:76–85
Swanson DL (1990) Seasonal variation in cold hardiness and peak rates of cold-induced thermogenesis in the dark-eyed junco (Junco hyemalis). Auk 107:561–566
Swanson DL (1991) Seasonal adjustments in metabolism and insulation in the dark-eyed junco. Condor 93:538–545
Swanson DL (2007) Seasonal metabolic variation in birds: functional and mechanistic correlates. In: Curr Ornithol, vol 17 (in press)
Swanson DL, Dean KL (1999) Migration-induced variation in thermogenic capacity in migratory passerines. J Avian Biol 30:245–254
Swanson DL, Olmstead KL (1999) Evidence for a proximate influence of winter temperatures on metabolism in passerine birds. Physiol Biochem Zool 72:566–575
Swanson DL, Weinacht DP (1997) Seasonal effects on metabolism and thermoregulation in northern bobwhite. Condor 99:478–489
Terblanche JS, Janion C, Chown SL (2007) Variation in scorpion metabolic rate and rate–temperature relationships: implications for the fundamental equation of the metabolic theory of ecology. J Evol Biol 20:1602–1612
Terroine EF, Trautmann S (1927) Influence de la température extérieure sur la production calorique des Homéothermes et loi des surfaces. Annales de Physiologie et de Physicochimie Biologique 3:422–457
Tieleman BI, Williams JB (2000) The adjustment of avian metabolic rates and water fluxes to desert environments. Physiol Biochem Zool 73:461–479
Tieleman BI, Williams JB, Buschur ME, Brown CR (2003) Phenotypic variation of larks along an aridity gradient: are desert birds more flexible? Ecology 84:1800–1815
Tieleman BI, Williams JB, Visser GH (2004) Energy and water budgets of larks in a life history perspective: parental effort varies with aridity. Ecology 85:1399–1410
Veghte JH (1964) Thermal and metabolic responses of the gray jay to cold stress. Physiol Zool 37:316–328
Veghte JH (1975) Thermal exchange between the raven (Corvus corax) and its environment. PhD. Thesis, University of Michigan, Ann Arbor
Vezina F, Jalvingh K, Dekinga A, Piersma T (2006) Acclimation to different thermal conditions in a northerly wintering shorebird is driven by body mass-related changes in organ size. J Exp Biol 209:3141–3154
Vézina F, Williams TD (2005) Interaction between organ mass and citrate synthase activity as an indicator of tissue maximal oxidative capacity in breeding European starlings: implications for metabolic rate and organ mass relationships. Funct Ecol 19:119–128
Wallgren H (1954) Energy metabolism of two species of Emberiza. Acta Zoologica Fennica 84:5–110
Weathers WW (1979) Climatic adaptation in avian standard metabolic rate. Oecologia 42:81–89
Weathers WW, Caccamise DF (1978) Seasonal acclimatization to temperature in monk parakeets. Oecologia 35:173–183
Weathers WW, Sullivan KA (1993) Seasonal patterns of time and energy allocation by birds. Physiol Zool 66:511–536
West GC (1972a) The effect of acclimation and acclimatization on the resting metabolic rate of the common redpoll. Comp Biochem Physiol 43A:293–310
West GC (1972b) Seasonal differences in resting metabolic rate of Alaskan ptarmigan. Comp Biochem Physiol A 41:867–876
White CR, Blackburn TM, Martin GR, Butler PJ (2007) The basal metabolic rate of birds is associated with habitat temperature and precipitation, not productivity. Proc R Soc B 274:287–293
White CR, Seymour RS (2004) Does basal metabolic rate contain a useful signal? Mammalian BMR allometry and correlations with a selection of physiological, ecological, and life-history variables. Physiol Biochem Zool 77:929–941
Wiersma P, Muñoz-Garcia A, Walker A, Williams JB (2007) Tropical birds have a slow pace of life. Proc Natl Acad Sci USA 104:9340–9345
Wijnandts H (1984) Ecological energetics of the long-eared owl (Asio otus). Ardea 72:1–92
Wikelski M, Spinney L, Schelsky W, Scheuerlein A, Gwinner E (2003) Slow pace of life in tropical sedentary birds: a common-garden experiment on four stonechat populations from different latitudes. Proc R Soc Lond B 270:2383–2388
Williams JB, Tieleman BI (2000) Flexibility in basal metabolic rate and evaporative water loss among hoopoe larks exposed to different environmental temperatures. J Exp Biol 203:3153–3159
Acknowledgments
I thank Ian Hume for inviting me to write this review. The manuscript benefitted greatly from a discussion with Steven Chown, and from the constructive comments of Craig Willis and an anonymous reviewer. The work was facilitated by funding from the DST/NRF Centre of Excellence at the Percy FitzPatrick Institute and the University of the Witwatersrand.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
McKechnie, A.E. Phenotypic flexibility in basal metabolic rate and the changing view of avian physiological diversity: a review. J Comp Physiol B 178, 235–247 (2008). https://doi.org/10.1007/s00360-007-0218-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-007-0218-8