Abstract
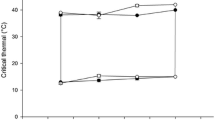
We investigated the temperature dependence of some physiological parameters of common eelpout (Zoarces viviparus) from different locations (North Sea, Baltic Sea and Norwegian Sea) on acclimation temperature (3 °C and 12 °C) and acute temperature variation. The lethal limit of 12 °C-acclimated eelpout was determined as the critical thermal maximum [loss of equilibrium (LE) and onset of muscular spasms (OS)] and it was found to be 26.6 °C for LE and 28.8 °C for OS for all populations. However, these parameters do not have any relevant ecological interpretation. We therefore investigated the effect of gradually increased water temperature on standard metabolic rate (measured as resting oxygen consumption Mo2) and critical oxygen concentration ([O2]c) of eelpouts. Acclimation to low temperature (3 °C) resulted in partial compensation of Mo2, paralleled by a decrease of activation energy for Mo2 (from 82 kJ mol–1 at 12 °C to about 50 kJ mol–1 at 3 °C) in North Sea and Baltic Sea eelpouts. At the same time, Norwegian eelpout showed no acclimation of oxygen demand to warm temperature (12 °C) at all. The scope for eelpout aerobic metabolism shrank considerably with increased acclimation temperature, as [O2]c approached water oxygen concentrations. At 22.5±1 °C the [O2]c reached air saturation, which is equivalent to the upper critical temperature (TcII) and at this temperature the aerobic scope for the metabolism completely disappeared. In line with previous insight, the comparative analysis of the temperature dependence of Mo2 of Z. viviparus from different populations suggests that a pejus (sub-critical) temperature for this species is about 13–15 °C. In conclusion, the capacity to adjust aerobic metabolism relates to thermal tolerance and the bio-geographical distribution of the species. Global warming would thus be likely to cause a shift in the distribution of this species to the North.








Similar content being viewed by others
Notes
For information on the BEEP project, please see: http://beep.1ptc.u-bordeaux.fr/_WP4.asp
For information on the ESB project, please see:http://www.umweltbundesamt.de/uba-info-daten-e/daten-e/umweltprobenbank-des-bundes.htm
For information on the CLICOFI project, please see: http://www.awi-bremerhaven.de/ECOLOGY/CLICOFI/
Abbreviations
- CTMax :
-
critical thermal maximum
- Fiv :
-
factor of inside volume
- G T :
-
total oxygen conductance
- LE :
-
loss of equilibrium
- LT 50 :
-
time period of exposure when mortality reaches 50%
- M O2 :
-
rate of oxygen consumption
- M O2 o :
-
rate of oxygen consumption at 0 °C
- [O 2 ] c :
-
critical oxygen concentration
- OS :
-
onset of muscular spasms
- Tc :
-
critical temperature
- Tc ll :
-
upper critical temperature
- Tp :
-
pejus temperature
References
Babkov AI (1982) A short hydrological characteristic of Chupa Inlet in the White Sea. In: Kulakowski EE (ed) Ecological investigations of species perspective for aquaculture in the White Sea. Zoological Institute Leningrad, Leningrad, USSR, pp 94–98
Bohnecke G, Dietrich G (1951) Monatskarten der Oberflächentemperaturen for die Nord—und Ostsee und die angrenzenden Gewässer. Hamburg, Deutscher Hydrographisches Institut Hamburg
Boutilier RG, St-Pierre J (2000) Surviving hypoxia without really dying. Comp Biochem Physiol A 126:481–490
Brodte E (2001) Wachstum und Fruchtbarkeit der Aalmutterarten Zoarces viviparus (Linn?) und Pachycara brachycephalum (Pappenheim) aus unterschiedlichen klimatischen Regionen. Fach Biologie University Bremen, Alfred—Wegener—Institut f?r Polar- und Meeresforschung, Bremerhaven
Christiansen FB, Nielsen BV, Simonsen V (1981) Genetical and morphological variation in the eelpout Zoarces viviparus. Can J Genet Cytol 23:163–172
Christiansen FB, Nielsen VH, Simonsen V (1988) Genetics of Zoarces populations. 15. Genetic and morphological variation in Mariager Fjord. Hereditas 109:99–112
Cossins AR, Bowler K (1987) Temperature biology of animals. Chapman and Hall, London
Fischer P, Rademacher K, Kils U (1992) In situ investigations on the respiration and behaviour of the eelpout Zoarces viviparus under short-term hypoxia. Mar Ecol Prog Ser 88:181–184
Fonds M, Jaworski A, Iedema A, Puyl PVD (1989) Metabolism, food consumption, growth and food conversion of shorthorn sculpin (Myoxocephalus scorpius) and eelpout (Zoarces viviparus). I. C. E. S., Demersal Fish Committee G:31:1–10
Frederich M, Pörtner HO (2000) Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squina. Am J Physiol Reg Int Comp Physiol 279:R1531–R1538
Frydenberg O, Simonsen V (1973) Genetics of Zoarces populations. 5. Amount of protein polymorphism and degree of genic heterozygosity. Hereditas 75:221–231
Herreid CF (1980) Hypoxia in invertebrates. Comp Biochem Physiol A 67:311–320
Hjorth JP, Simonsen V (1975) Genetics of Zoarces populations. 8. Geographic variation common to the polymorphic loci HbI and EstIII. Hereditas 81:173–184
Johnston IA (1985) Temperature adaptation of enzyme function in fish muscle. In: Laverack MS (ed) Physiological adaptations of marine animals. Company of Biologists, pp 95–122
Johnston IA, Walesby NJ (1977) Molecular mechanisms of temperature adaptation in fish myofibrillar adenosine triphosphates. J Comp Physiol B 119:195–206
Klimant I, Kuhl M, Glud RN, Holst G (1997) Optical measurement of oxygen and temperature in microscale: strategies and biological applications. Sensors Actuators 38–39 B:29–37
Kristoffersson R, Oikari A (1975) Notes on the biology of the eelpout, Zoarces viviparus ( L.), in the brackish water of Tvärminne, Gulf of Finland. Annales zoologici fennici 12:143–147
Lutterschmidt WI, Hutchison VH (1997a) The critical thermal maximum: data to support the onset of spasms as the definitive end point. Can J Zool 75:1553–1560
Lutterschmidt WI, Hutchison VH (1997b) The critical thermal maximum: history and critique. Can J Zool 75:1561–1574
Motulsky H (1999) Analyzing data with GraphPad Prism. GraphPad Software, San Diego, Calif.
O'Brien J, Dahlhoff E, Somero GN (1991) Thermal resistance of mitochondrial respiration: hydrophobic interactions of membrane proteins may limit thermal resistance. Physiol Zool 64:1509–1526
Pörtner HO (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Pörtner HO (2002a) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A 132:739–761
Pörtner HO (2002b) Physiological basis of temperature-dependent biogeography: trade-offs in muscle design and performance in polar ectotherms. J Exp Biol 205:2217–2230
Pörtner HO, Grieshaber MK (1993) Critical Po2(s) in oxyconforming and oxyregulating animals: gas exchange, metabolic rate and the mode of energy production. In: Bicudo JEPW (ed) The vertebrate gas transport cascade: adaptations to environment and mode of life. CRC, Boca Raton, pp 330–357
Pörtner HO, Hardewig I, Sartoris FJ (1998) Energetic aspects of cold adaptation: critical temperatures in metabolic, ionic and acid-base regulation? In: Pörtner HO, Playle RC (eds) Cold ocean physiology. Cambridge University Press, Cambridge, pp 88–120
Pörtner HO, Berdal B, Blust R, Brix O, Colozimo A, De Wachter B, Giuliani A, Johansen T, Fischer T, Knust R, Lannig G, Naevdal G, Nedenes A, Nyhammer G, Sartoris FJ, Serendero I, Sirabella P, Thorkildsen S, Zakhartsev MV (2001) Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: developing a hypothesis for cause and effect relationship in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus). Continental Shelf Res 21:1975–1997
Precht H, Chritophersen J, Hensel H, Larcher W (1973) Temperature and life. Springer, Berlin Heidelberg New York
Ross LG, Ross B (1999) Anaesthetic and sedative techniques for aquatic animals. Blackwell, Oxford
Shelford VE (1913) Animal communities in temperate America. The University of Chicago Press, Chicago
Sokal RR, Rohlf FJ (1995) Biometry. The principles and practice of statistics in biological research. W.H. Freeman, New York
Sokolova IM, Pörtner HO (2001) Temperature effects on key metabolic enzymes in Littorina saxatilis and L. obtusata from different latitudes and shore levels. Mar Biol 139:113–126. DOI 10.1007/s002270100557
Sommer A, Klein B, Pörtner HO (1997) Temperature induced anaerobiosis in two populations of the polychaete worm Arenicola marina (L). J Comp Physiol B 167:25–35
Stoskopf MK (1992) Fish medicine. Saunders, Philadelphia
Ultsch GR, Ott ME, Heisler N (1980) Standard metabolic rate, critical oxygen tension, and aerobic scope for spontaneous activity of trout (Salmo gardneri) and carp (Cyprinus carpo) in acidified water. Comp Biochem Physiol A 67: 329–335
Van Dijk PLM, Tesch C, Hardewig I, Pörtner HO (1999) Physiological disturbances at critically high temperatures: A comparison between stenothermal antarctic and eurythermal temperate eelpouts (Zoarcidae). J Exp Biol 202:3611–3621
Yeager DP, Ultsch GR (1989) Physiological regulation and conformation: a BASIC program for the determination of critical points. Physiol Zool 62:888–907
Zielinski S, Lee PG, Pörtner HO (2000) Metabolic performance of the squid Lolliguncula brevis (Cephalopoda) during hypoxia: an analysis of the critical Po 2 . J Exp Mar Biol Ecol 243:241–259
Acknowledgements
A contribution to the ELOISE project: Effect of climate induced temperature change on marine coastal fishes (CLICOFI), funded by the European Union program "Climate and environment", contract No. ENV4-CT97–0596. The experiments comply with the current laws of the Belgium and Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier
Rights and permissions
About this article
Cite this article
Zakhartsev, M.V., De Wachter, B., Sartoris, F.J. et al. Thermal physiology of the common eelpout (Zoarces viviparus). J Comp Physiol B 173, 365–378 (2003). https://doi.org/10.1007/s00360-003-0342-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-003-0342-z