Abstract
Purpose
Urethral strictures are a common disease of the lower urinary tract in men. At present, the use of buccal mucosa is the method of choice for long or recurrent strictures. However, autologous tissue-engineered grafts are still under investigation for reconstructive urological surgery. The aim of this pilot study was to evaluate the use of human urothelial cells (HUC) seeded on bovine collagen type I-based cell carriers (CCC) in an animal model and to evaluate short-term outcome of the surgical procedure.
Methods
Four male Göttingen minipigs were used with immunosuppression (cyclosporine A) for this pilot xenograft study. HUC obtained from human benign ureteral tissue were stained by PKH26 and seeded on a collagen cell carrier (CCC). Seven weeks after urethral stricture induction and protective vesicostomy, cell-seeded CCC was implanted in the urethra with HUC luminal and antiluminal, respectively. After two weeks animals were euthanized, urethrography and histological assessment were performed.
Results
Surgery was technically feasible in all minipigs. Stricture was radiologically established 7 weeks after induction. CCC was visible after two weeks and showed good integration without signs of inflammation or rejection. In the final urethrography, no remaining stricture could be detected. Near porcine urothelium, PKH26-positive areas were found even if partially detached from CCC. Although diminished, immunofluorescence with pankeratin, CK20, E-cadherin and ZO-1 showed intact urothelium in several areas on and nearby CCC.
Conclusion
Finally, this study demonstrates that the HUC-seeded CCC used as a xenograft in minipigs is technically feasible and shows promising results for further studies.
Similar content being viewed by others
Introduction
Urethral strictures are a common disease of the lower urinary tract in men and reflect a challenging problem in urology [1, 2].
Due to the high risk of stricture recurrence, often further surgeries are necessary. In these cases, complex reconstructive surgery with the use of free grafts is needed [3, 4]. At this point, preputial flap or oral mucosa is used for reconstruction of the urethra with high success rates [4, 5]. However, the limited donor site availability and the morbidity in multiple used grafts especially in patients with nicotine abuse may compromise surgical feasibility and clinical outcome in urethral stricture patients [6]. Furthermore, due to high risk for recurrence reconstructive urology focused mainly on tissue-engineering techniques in the last decade. Nowadays, there are various different possibilities as unseeded and single cell-seeded scaffolds or cultured epithelium [7].
In the presented pilot xenograft project, a bovine collagen type I-based cell carrier (CCC) was used. This matrix should improve the adhesion of the seeded cells and facilitate the proliferation and functionality of the implanted human urothelial cells (HUC). Furthermore, the stability of the matrix should simplify surgical integration into the urethral defect. After surgical stricture induction in four male minipigs, urethroplasty was performed using HUC seeded on ultrathin CCC (20 μm).
The aim of this pilot study was to evaluate the xenogenic use of HUC in an animal model, the handling of the seeded CCC and the short-term outcome of the surgical procedure.
Methods
For this study, a male porcine urethra was used, due to its physiologically comparability to the human male urethra [8]. Therefore, four male Göttingen minipigs (castrated, Ellegaard, Dalmose, Denmark) were used for this pilot xenograft study. Weight at first surgery was 20–25 kg. Housing of animals was performed according to the animal ethics committee Tuebingen protocol (CU 2/12). HUC were isolated from human ureter tissue specimens of an adult female patient obtained during laparoscopic nephrectomy due to a non-functional kidney (institutional ethics committee approval no. 379/2010BO2).
Minipig surgeries
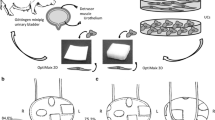
All four minipigs have undergone two surgeries under general anaesthesia before being killed 9 weeks after first surgery. In the first intervention, a urethral stricture was induced after preparation of the penis by a needle inserted through the penile part of the urethra and spongious body [9, 10]. Afterwards, stricture was induced by monopolar thermocoagulation (Fig. 1a). Thereafter, similar to urethral reconstruction in humans, a protective vesicostomy was established to guarantee free and continuous flow of the urine and to avoid wound-healing disorders of the penile urethra (urine paravasation) as well as cell detachment from CCC-implants for the next 7 weeks (Fig. 1b).
Surgery. a In a first step, stricture was induced by transurethral needle insertion and consecutive thermocoagulation. b In the same surgery, vesicostomy was performed. c The urethral stricture was established and documented 6 weeks after first surgery before CCC implantation (arrow shows stricture). d Implantation of the HUC seeded on CCC. e Graphical visualization of the porcine urethra (black line) showing the luminal (left) and antiluminal (right) placement of the human urethral cells (red line) on the collagen cell carrier (blue line) using the ventral onlay technique for porcine urethral reconstruction
In the second surgical intervention after radiological evaluation of the stricture (Fig. 1c), a ventral onlay urethroplasty with stratified HUC–CCC-implants (static culture conditions) was performed (Fig. 1d). The strictured part of the urethra was opened longitudinally; afterwards, CCC was adapted in size, inserted and fixed by a bilateral 6.0 polyglactin running suture. The spongy tissue was closed to cover the implant. In two minipigs, HUC seeded on the CCC were applied luminal, whereas in two pigs the layer with HUC was implanted anti-luminal (Fig. 1d) to investigate the behaviour of the urothelial cells regarding possible differences in cell detachment and migration (Fig. 1e). Non-absorbable sutures were used in the first and second surgery to mark the induced stricture and afterwards the reconstructed urethra, respectively. The distance of the marker sutures was about 2 cm. We avoided the use of a transurethral catheter due to the risk of cell detachment, irritation during movement and infection. After two weeks, all animals were euthanized according a standardized veterinarian protocol and urethral tissue was sampled for histological and immunohistological analysis. Urethrography was performed at every intervention to show the stricture formation or absence of a urethral stricture, respectively. Due to the use of xenogeneic material all minipigs had immunosuppressive therapy with cyclosporin A throughout the whole observation period started one week before first surgery.
Isolation and seeding of HUC on collagen cell carrier
Urothelial cells were sampled and cultured under sterile conditions as described previously (CnT-07; CELLnTEC Advanced Cell Systems, Bern, Switzerland) [11]. The standardized CCC used derived from pure bovine collagen type I and was provided by Viscofan BioEngineering (Weinheim, Germany). For this study, cells were seeded in confluence (3–4 × 105 HUC/cm2) on each CCC insert. The following day, PKH26 (Sigma-Aldrich, St. Louis, MO, USA) labelling of the attached cells was performed and differentiation was induced. Afterwards HUC cultures were stratified (CELLnTEC Advanced Cell Systems, Bern, Switzerland) for 6 or 7 days with replacement of differentiation medium three times a week and then used for urethral reconstruction in four minipigs. Finally, labelled cells were visualized by fluorescence microscopy.
Tissue processing
After killing the animals, the urethras were extracted. Haematoxylin and eosin (HE) staining of cross sections was performed to evaluate integration of the implanted cells, CCC degradation and inflammation. In addition, tissue samples were even investigated by immunofluorescence to show expression of epithelial differentiation and cell–cell contact markers. Therefore, different mouse antibodies were used according to the user manual: pankeratin (1/100; Millipore), CK-20 (1/50; Dako, Hamburg, Germany), E-cadherin (1/100; Dako), and ZO–1 (1/40; Invitrogen).
Results
Minipig surgery
In all minipigs surgery with preparation of the penile urethra, stricture induction by transurethral needle insertion and implantation of the CCC was technically feasible. Performing a protective vesicostomy represents a quick, well tolerated and necessary procedure in this animal model. All minipigs showed no local or functional complications as infection, wound-healing disorder or stenosis of to the vesicostomy (Fig. 1b). Performance of a retrograde urethrogram by insertion of 8 French transurethral catheter showed a significant stricture development after monopolar stricture induction (Fig. 1a, c). Stricture length was estimated to range from 1 to 1.5 cm in between the marker sutures. Accordingly, oval-shaped implants estimated between 1.5 and 2 cm in length and maximum width of 0.5–1.0 cm have been used. Narcosis and perioperative treatment was tolerated well without any signs of behavioural disorders. The final retrograde urethrography performed two weeks after urethral reconstruction revealed no radiological evident stricture recurrence in all four pigs. However, although all four urethras showed a complete histological integrity one clearly visible paravasation was found in minipig number 2 (Fig. 3a).
Histological analysis: HE, native/PKH26
In all minipigs, the CCC, even still degrading, was visible after two weeks and showed good integration without signs of inflammation or rejection. In three pigs, the CCC was located near to the luminal wall of the urethra with direct contact in some areas. In one animal with HUC applied luminal (pig number 2), the CCC was more distant from the urethra.
After two weeks, large areas of red PKH26 fluorescence with different intensity were found in all four minipigs (Fig. 2). PKH26 fluorescence was detected almost adjacent to the lumen but partially separated from the CCC. In the two minipigs (pig number 1 and 3) with the matrix applied antiluminally, the porcine urothelium was not in a direct connection with the placed HUC. In this case, PKH26 fluorescences were nearly divided from the CCC in most of the areas. Furthermore, PKH26-positive areas were lower near the porcine urethra with weaker intensity compared to pigs where HUC were implanted luminally (Fig. 2). In minipig number 2 and 4 with HUC applied luminally, PKH26 fluorescence was detected close to the regenerated porcine urothelium (Fig. 2). In minipig number 2, PKH26 fluorescences were found adjacent to the porcine urethra even though the CCC was distant to the initial implantation area. However, a definitive PKH26 cell membrane labelling of the human implanted cells with regard to double staining with urothelial markers was not possible.
In all minipigs, the urethral lumen was completely intact (Fig. 3b). In regions with implanted CCC, the urothelial layer seemed to be thinner in minipig 1–3 compared to other areas representing the regenerated part of the porcine urethra (Fig. 3b, arrow).
a Retrograde urethrography 2 weeks after urethral reconstruction with human urothelial cells seeded on a collagen cell carrier showing no radiological urethral stricture recurrence in all pigs. A visible paravasation of contrast medium was found in Pig 2. b Cross sections of the porcine urethra (Pig 1–4) showing complete integrity of the lumen and regenerative host urothelium (black arrows). Collagen cell carrier (CCC) visible in Pig 1, 3 and 4 (asterisks)
Immunofluorescence
For verification of the urothelium transplant, fluorescence of pankeratin, CK-20, E-cadherin and ZO-1 was used. All antigens for epithelial phenotype and cell–cell junctions were detected in the native urothelium indicating unimpaired cell layers. Tissues were screened for antibody fluorescence at the transplanted area of the urethra.
Pankeratin-positive areas were found predominantly in native urothelium areas with few pankeratin-positive and PKH26-positive overlays. Nevertheless, pankeratin staining showed intact urothelium on the matrix (Fig. 4a, white line) 2 weeks after implantation.
Immunofluorescence analysis of implanted cells. a AE1AE3 pankeratin staining demonstrated intact urothelium on the CCC (white line) 2 weeks after implantation. b Fluorescence of CK-20 and PKH26 (b). c, d Positive fluorescence of ZO-1 (c) and E-cadherin (d) demonstrates functional cell–cell junctions in tissue-engineered urothelium
E-cadherin was clearly positive in urethral tissue showing intact cell adhesions within the tissue (Fig. 4d). CK20 and PKH26 should show the final differentiation of the implanted urothelial cells due to slight double fluorescence (Fig. 4b). However, a final statement about the differentiation of the implanted HUC was not possible in in vivo samples. Apart from integral urothelium, weak ZO-1-positive and PKH26-positive spots revealed functional cell–cell junctions of the CCC urothelium implant (Fig. 4c).
Discussion
The aim of the presented xenogeneic pilot study was to evaluate the use of HUC in an animal model, the handling of the seeded CCC and the short-term outcome of the surgical procedure. Comparability of urethral stricture development after thermocoagulation between the large animal model with minipigs and urethral stricture tissue in male humans has been demonstrated by our group in the past. Stricture length was comparable to our previous studies [9].
When looking at the functional outcome complete integrity of the urethral lumen has been found two weeks after implantation of the CCC. Despite the fact that CCC was not always completely attached to the porcine urethra, even if thinner, the porcine urothelium was microscopically intact in the implantation area. This may happen due to the possible vector function of the CCC inducing regeneration processes of the porcine urothelium with high turnover rates of the cells. Negative influence or adverse effects have not been identified in this study. Furthermore, regeneration can be supported through soluble factors and paracrine effects, which can pass through the semipermeable CCC membrane [12, 13]. However, due to the reported immunohistological findings a final conclusion whereby such processes were induced was not possible.
Antiluminal implanted HUC showed a stronger PKH26 detachment with a higher displacement from the CCC leading to the suggestion that luminal application of the urothelial cells should be used in further studies. In the clinical use in most of the cases, a transurethral catheter is placed after urethroplasty. This might influence luminally implanted cells due to mechanical irritation. In this animal model, no catheter was used after implantation of the CCC to avoid this effect. In addition, the direct contact of luminally implanted cells with the porcine urothelium might allow higher turnover rates leading to a better regeneration of the urethra. Based on our findings, luminal application of the HUC is considered in this animal model. However, in both groups (HUC luminal and antiluminal), a complete regeneration of the porcine urethra was observed. This might be enabled by the semipermeable characteristic of the used CCC. In the area of porcine urothelium, PKH26 fluorescences were found. Generally, immunofluorescence was detectable in the xenograft model with partly weak intensity. Detachment and weaker immunofluorescence might be explained by the fact that human cells were used in an animal model. However, even if PKH26 fluorescence was detectable in several areas near the urothelium, a definite cell membrane labelling of the human implanted cells was not possible. This can be explained by eventual interactions between human and porcine urothelial cells as well as macrophage-induced processes, degeneration of human cells and thus a spread of the PKH26 stain [14]. Despite the variable results of human cell detection, PKH26 staining is still an encouraging and feasible tool, which needs to be investigated in further studies.
Pankeratin immunofluorescence was of weak intensity with a low number of overlays with PKH26 areas. Hence, a clear demonstration of remaining vital human cells was not possible. The fact that adequate cyclosporine levels were measured at time of scarification excludes an immunological failure. Nevertheless, the use of urothelial cells in reconstructive surgery should be considered. Urothelial cells show functional characteristics as their uroplakin expression, which guarantees urothelial stability and resistance to urine, plays an important role in wound healing and inflammatory processes [15]. In addition, urothelial cells can be collected not only in form of biopsies but also by bladder washings which is a less invasive procedure with a low complication rate [16]. The fact that urothelial cell-seeded implants have similar histological appearance as native urethral tissue as shown by Raya-Rivera et al. [17] encourages the thesis that autologous tissue for reconstructive surgery might be promising.
Main limitation of this study is the low number of animals used for this pilot trial. Therefore, further studies with a larger number of minipigs are needed. Further limitations of the presented study include the absent use of CCC without any cells as well as a reconstruction without any graft simulating a sham model. We decided to start with a small study cohort to justify further experiments in larger cohorts with regard to ethical aspects, maintenance costs and care, especially in a large animal model. Also, the onlay technique may not offer an ideal wound bed in sense of highly vascularized tissue as reported in other studies where cellularized grafts were used [7].
However, the used CCC seems to increase the stability of the cell-seeded implants and may support survival and growth of urothelial cells leading to a complete regeneration of the porcine urethra.
Conclusion
Finally, this study demonstrates that the HUC-seeded CCC used as a xenograft in minipigs is technically feasible showing promising results for further studies.
References
Barbagli G, Palminteri E, Lazzeri M, Guazzoni G (2003) Anterior urethral strictures. BJU Int 92(5):497–505
Gozzi C, Tritschler S, Bastian PJ, Stief CG (2008) Management of urethral strictures. Der Urologe Ausg A 47(12):1615–1622. doi:10.1007/s00120-008-1903-2
Rodder K, Olianas R, Fisch M (2006) [Urethral strictures–operative strategy]. Der Urologe Ausg A 45 (4):499–511; quiz 512–493. doi:10.1007/s00120-006-1031-9
Engel O, Fisch M (2010) Urethral reconstruction after failed primary surgery. Der Urologe Ausg A 49(7):822–826. doi:10.1007/s00120-010-2315-7
Bhargava S, Chapple CR (2004) Buccal mucosal urethroplasty: is it the new gold standard? BJU Int 93(9):1191–1193. doi:10.1111/j.1464-410X.2003.04860.x
Sinha RJ, Singh V, Sankhwar SN, Dalela D (2009) Donor site morbidity in oral mucosa graft urethroplasty: implications of tobacco consumption. BMC Urol 9:15. doi:10.1186/1471-2490-9-15
Mangera A, Chapple CR (2013) Tissue engineering in urethral reconstruction: an update. Asian J Androl 15(1):89–92. doi:10.1038/aja.2012.91
Idzenga T, Pel JJ, van Mastrigt R (2006) A biophysical model of the male urethra: comparing viscoelastic properties of polyvinyl alcohol urethras to male pig urethras. Neurourol Urodyn 25(5):451–460. doi:10.1002/nau.20282
Seibold J, Selent C, Feil G, Wiedemann J, Colleselli D, Mundhenk J, Gakis G, Sievert KD, Schwentner C, Stenzl A (2012) Development of a porcine animal model for urethral stricture repair using autologous urothelial cells. J Pediatr Urol 8(2):194–200. doi:10.1016/j.jpurol.2011.02.001
Sievert KD, Selent-Stier C, Wiedemann J, Greiner TO, Amend B, Stenzl A, Feil G, Seibold J (2012) Introducing a large animal model to create urethral stricture similar to human stricture disease: a comparative experimental microscopic study. J Urol 187(3):1101–1109. doi:10.1016/j.juro.2011.10.132
Feil G, Christ-Adler M, Maurer S, Corvin S, Rennekampff HO, Krug J, Hennenlotter J, Kuehs U, Stenzl A, Sievert KD (2006) Investigations of urothelial cells seeded on commercially available small intestine submucosa. Eur Urol 50(6):1330–1337. doi:10.1016/j.eururo.2006.05.041
Hennig C, Theuring F (1990) Physiologic regeneration of rat urothelium after pulse and continual labeling with 3H-thymidine at various ages. Acta Histochem Suppl 39:263–266
Bouhout S, Chabaud S, Bolduc S (2016) Organ-specific matrix self-assembled by mesenchymal cells improves the normal urothelial differentiation in vitro. World J Urol 34(1):121–130. doi:10.1007/s00345-015-1596-2
Zhu L, Fang Y, Liu Z, Wang P, Wang Y, Xu H (2008) Rabbit anti-human leukocyte polyclonal antibody inhibits xenogeneic cell-mediated immune responses. Transpl Proc 40(8):2760–2763. doi:10.1016/j.transproceed.2008.07.111
Katnik-Prastowska I, Lis J, Matejuk A (2014) Glycosylation of uroplakins: implications for bladder physiopathology. Glycoconj J 31(9):623–636. doi:10.1007/s10719-014-9564-4
Nagele U, Maurer S, Feil G, Bock C, Krug J, Sievert KD, Stenzl A (2008) In vitro investigations of tissue-engineered multilayered urothelium established from bladder washings. Eur Urol 54(6):1414–1422. doi:10.1016/j.eururo.2008.01.072
Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A (2011) Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 377(9772):1175–1182. doi:10.1016/S0140-6736(10)62354-9
Acknowledgements
The study was supported by Viscofan Bioengineering, a business unit of Naturin Viscofan GmbH, Weinheim, Germany. We want to thank Prof. Dr. Wilhelm K. Aicher for his help in evaluation of experimental data.
Authors’ contribution
SA analysed the data, wrote and edited the manuscript. MV was involved in protocol/project development, data collection or management. AK analysed the data. SM was involved in data collection or management, data analysis. LG, JM and LD contributed to data collection or management. SB developed the protocol/project, analysed the data and edited the manuscript. AS and K-DS were involved in protocol/project development, manuscript writing/editing. BA was involved in data collection or management, data analysis, manuscript writing/editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. (Institutional ethics committee approval no. 379/2010BO2). All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Aufderklamm, S., Vaegler, M., Kelp, A. et al. Collagen cell carriers seeded with human urothelial cells for urethral reconstructive surgery: first results in a xenograft minipig model. World J Urol 35, 1125–1132 (2017). https://doi.org/10.1007/s00345-016-1959-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-016-1959-3