Abstract
Growth of strongly textured \(\mathrm{FeCO}_{3}\) thin films on substrates was achieved with ultrashort-pulsed laser deposition using 810-nm, 46-fs ablation pulses. The crystallinity and composition were verified with X-ray diffraction and Raman spectroscopy. Using Mössbauer spectroscopy, it is shown that the deposited \(\mathrm{FeCO}_{3}\) thin films possess the film quality required for application in research of nuclear quantum optics. It is found that a relatively low substrate temperature is crucial for growing a strongly textured film of \(\mathrm{FeCO}_{3}\) while avoiding decomposition of \(\mathrm{FeCO}_{3}\) into \(\mathrm{Fe}_{2}\mathrm{O}_{3}\) and \(\mathrm{CO}_{2}\). This supports the importance of the use of ultrashort-pulsed laser deposition in providing adatoms with high mobility for attaining good crystallinity. The surface morphology was characterized by surface profilometry, scanning electron microscopy and atomic force microscopy. It is found to be significantly affected by changing the ablation laser parameters, including laser fluence, pulse duration, and on-target spot size. The results show that the peak deposition flux must be below approximately 0.03 nm/pulse in order to grow a flat film.
Similar content being viewed by others
1 Introduction
Crystalline FeCO3 is an important carbonate material which has many interesting applications in various scientific fields, such as redox geochemistry, iron biomineralization, corrosion science, Fe/CO2 fuel cells, geological CO2 sequestration, and nuclear quantum optics [1–3]. In the field of nuclear quantum optics, the recent pioneering works on nuclear-level-mixing-induced transparency have received great attention [2–4]. In these experiments, the samples used were monocrystalline FeCO3 films obtained from natural mineral siderite. The presence of substitutional impurities such as Mg and Ca is crucial for inducing nuclear level anti-crossing that leads to reduced absorption (increased transparency). However, in these natural sample, neither the percentage of the impurities can be controlled, nor the Fe component can be isotope-enriched to enhance signal intensity. Artificial synthesis of FeCO3 single crystal has been demonstrated by using hydrothermal method [5], but none has been used in the experiments. Besides the difficulty in obtaining a sample with sufficiently large area and specified crystal orientation, the concentration of impurities cannot be easily controlled as a result of segregation in hydrothermal synthesis. In contrast, deposition methods based on layer-by-layer growth on a suitable substrate seem to be a good way to overcome these problems. However, to the best of our knowledge there has not been any report of growth of FeCO3 film on a substrate, neither monocrystalline nor polycrystalline. The difficulty lies in the conflicting requirements of a sufficiently high temperature to grow a monocrystalline film and a moderate temperature (about 250 °C [6, 7]) to keep FeCO3 from decomposing into Fe2O3 (or Fe3O4) and CO2. In fact, to the best of our knowledge there has not been any report on successful growth of any kind of carbonate monocrystalline film on a substrate, probably all due to the same decarbonation problem.
Pulsed laser deposition (PLD) has been shown to be a versatile technique for growing thin films of a wide range of materials, from single atomic layer to quasi-bulk crystalline films [8]. Its great capability lies in its ability to nonthermally and stoichiometrically transfer material from a multi-element target onto a substrate on a layer-by-layer basis. Properties of films, such as crystallinity and morphology, can be conveniently controlled by varying control parameters such as laser fluence, on-target laser spot size, target-to-substrate distance, substrate temperature, and substrate type. This allows one to obtain the optimal parameters for depositing films that meet the requirement of specific applications. In particular, ultrashort-pulsed laser deposition (uPLD) has drawn much attention recently [9]. The uPLD provides more tunability in the ionization state and ion momentum of the plasma plume, offering more control factors for film growth [9–11]. An important feature of uPLD is its capability of growing crystalline film at a lower substrate temperature than using PLD or other deposition methods, because uPLD can provide the adatoms with higher mobility by raising the momenta of the ions and atoms in the plasma plume and thus the transient temperature of the substrate surface. With a lower substrate temperature the problem of decarbonation of FeCO3 deposited on the substrate may be circumvented.
In this paper, we report on the growth of strongly textured FeCO3 thin films on substrates by using ultrashort-pulsed laser deposition. Taking advantage of the unique capability of uPLD to provide sufficient mobility for adatoms just deposited while keeping the substrate temperature at moderate value to avoid decomposition of existing layers, growth of FeCO3 (104) strongly textured film on a Al2O3 (104) substrate was achieved. Using Mössbauer spectroscopy, it is shown that the FeCO3 films produced under the optimal deposition condition have the crystalline quality required for application in research of nuclear quantum optics. Moreover, a detailed study of the dependence of crystallinity and surface morphology of grown FeCO3 thin films on various uPLD parameters was also conducted. It was found that while the crystallinity of FeCO3 thin films does not change significantly with variation of ablation laser parameters such as energy fluence, pulse duration, and on-target spot area by a factor of 10, the surface morphology depends critically on these parameters and thus the latter must be chosen with care in order to obtain a film with good surface flatness.
2 Experimental
The experimental setup for uPLD is shown in Fig. 1. The uPLD was carried out in a chamber evacuated down to \({<}2\times 10^{-4}~\mathrm{torr}\). A 810-nm, 10-Hz Ti:sapphire laser system was used for this experiment [12]. The \(p\)-polarized ablation laser beam of 4-cm diameter propagated from the pulse-compression chamber to the uPLD chamber through a 1.6-mm-thick fused-silica window, and then it was focused with an f/7.5 off-axis parabolic mirror (OAP) onto a polycrystalline FeCO3 target at an incidence angle of 45°. It passed through an optical-quality glass window (1.1-mm-thick B270 window) in the shielding box before reaching the target. The group delay dispersion introduced by these windows could be compensated by adjusting the distance between the gratings in the pulse compressor. The self-phase modulation introduced by these windows was negligible and had no observable effect on the pulse duration and spectrum. The shielding box enclosed the target and substrate holders so that the ablated material did not coat the optical components inside the vacuum chamber. The drop in the laser fluence after growing a film (taking about two hours at the optimal condition) was less than a few percent, so its effect on the deposition condition could be neglected. After the deposition of each film the glass window in the shielding box was wiped clean with acetone. This restored the optical transmission of the glass window to its original value. The energy of the ablation pulse was controlled by an energy tuner composed of a half-wave plate and a thin-film polarizer, and the pulse duration of the ablation laser beam could be varied from 46 fs to 3 ps by detuning the distance between the gratings in the pulse compressor. The ablation beam has a Gaussian-like profile. The on-target laser spot size was tunable from to \(1~\mbox{mm} \times 1.4~\mbox{mm}\) in full width at half maximum (FWHM) by controlling the motorized translation stage under the OAP. A relayed-imaging system was used to monitor the on-target position and size of the ablation spot under vacuum, by detecting the part of the ablation beam that was reflected from the glass window in the shielding box.
FeCO3 powder purchased from City Chemical LLC was first pressed into a 1-cm-diameter 3-mm-thick pill at a pressure of \(8~\mbox{ton/cm}^{2}\), and then the pill was sintered in an oven at 100 °C for 4 hours. The resulting hard pill was used as the uPLD target, which was identified as polycrystalline FeCO3 with X-ray diffraction (XRD), as shown in Fig. 2(a). The photo of the FeCO3 uPLD target is shown in the inset in Fig. 2(a). The target was mounted on a carousel-type holder which contained five target mounts, and the target being ablated could be switched under vacuum via a computer controlled motor. The motorized target mounts were also controlled by the computer program to automatically rotate and translate after each laser shot. This was for providing a different location for the next ablation pulse, to avoid formation of large craters and thus change of on-target laser ablation parameters. A quartz crystal microbalance calibrated for FeCO3 was installed to monitor deposition rate and film thickness.
XRD spectra of uPLD target, (a), and of 2-μm FeCO3 films on glass substrates at substrate temperatures of (b) 25 °C, (c) 100 °C, and (d) 270 °C. The ablation laser beam for uPLD is of 810-nm central wavelength, \(1\mbox{-J/cm}^{2}\) fluence, 46-fs pulse duration, and on-target spot size. The target-to-substrate distance is 25 mm. The inset in (a) shows a photo of the uPLD target
The \(1\mbox{-cm} \times 1\mbox{-cm}\) substrates were mounted on another motorized carousel-type holder which contained six substrate mounts and which allowed under vacuum computer-controlled switching of the substrate for coating. Each mount had its own heater and thermocouple such that the temperature of each substrate could be independently programmed. The maximum substrate temperature was 750 °C. A mask was installed in front of the substrate holder to shield the substrates except the one being coated. The samples were characterized by using a powder X-ray diffractometer (X-ray \(\mathrm{wavelength} = 0.154~\mbox{nm}\)), a four-circle X-ray diffractometer (X-ray \(\mathrm{wavelength} = 0.154~\mathrm{nm}\)) and a Raman spectrometer for measuring crystalline structure and crystallinity. A surface profiler (tip radius of curvature ), a scanning electron microscope (SEM), and an atomic force microscope (AFM) (tip radius of curvature \({\le} 12~\mathrm{nm}\)) were used for analyzing the surface morphology of samples on various scales. A Mössbauer spectrometer with 57Co/Rh radioactive source was used to measure Mössbauer spectra of 57Fe in the grown films.
3 Results and discussion
As the first step, deposition of 2-μm-thick films on glass substrates was carried out at various substrate temperatures. The XRD spectra of the samples are shown in Figs. 2(b)–(d) respectively. The ablation laser fluence was \(1~\mbox{J/cm}^{2}\), the pulse duration was 46 fs, and the on-target laser spot size was . The target-to-substrate distance was 25 mm. For the films grown at substrate temperatures of room temperature and 100 °C their XRD spectra exhibit two prominent narrow peaks which match with the theoretical diffraction peaks of polycrystalline FeCO3 and that of the uPLD target used (Fig. 2(a)). For the film grown at a substrate temperature of 270 °C the intensities of FeCO3 XRD peaks are substantially lower compared to the previous cases, and a Fe2O3 peak shows up. The emergence of the Fe2O3 peak indicates that a portion of the FeCO3 film decomposes into Fe2O3 and CO2 at such a substrate temperature, consistent with the known property of FeCO3 [6, 7].
For growing a strongly textured film with preferred (104) orientation monocrystalline Al2O3 (104) substrate were used instead. This type of substrates was chosen in order to provide a small lattice mismatch between the film and the substrate. Both FeCO3 siderite and Al2O3 sapphire crystals belong to the hexagonal crystal system. For the normal plane of (104) the epitaxy condition can be represented by the arrangement of atoms along the [\({\bar{1}2\bar{1}0}\)] and [\({\bar{8}{4}{4}{3}}\)] directions. The interatomic distances of the film and the substrate in the [\({\bar{1}2\bar{1}0}\)] direction are 0.472 and 0.475 nm respectively, and thus the lattice mismatch ratio in this direction is \(0.66~\%\). The interatomic distances of the film and the substrate in the [\(\bar{8}{4}{4}{3}\)] direction are 2.440 and 2.301 nm respectively, and thus the lattice mismatch ratio in this direction is \(5.88~{\%}\). For this case, the target-to-substrate distance was increased to 40 mm, based on the preliminary results obtained with glass substrates, which showed that a smoother film can be produced with a lower deposition flux. The laser parameters were the same as those used in Fig. 2. The substrate temperature was varied from 100 to 300 °C. For annealing after completion of the deposition, the film was kept for 1 hour at the substrate temperature used during deposition and then decreased to the room temperature in 3 hours.
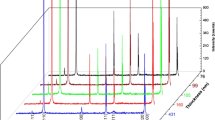
Figure 3 shows the XRD spectra of 2-μm FeCO3 films deposited on Al2O3 (104) substrates at various substrate temperatures. At a substrate temperature below 200 °C FeCO3 polycrystalline films were produced, similar to the results with glass substrates. For the films grown at a substrate temperature of 250 °C, as shown in Fig. 3(c), the XRD peak corresponding to FeCO3 (104) turns much stronger than for the films grown at lower substrate temperatures, whereas all other FeCO3 peaks vanish. This indicates that the grown film has only the (104) crystalline component. The strongly textured nature is verified by the sharp Raman peak shown in Fig. 4(a), which rules out the presence of amorphous domains. The inset in Fig. 4(a) shows the fit of the FeCO3 Raman peak with a Lorentzian curve. The peak width is 8 cm−1 in FWHM, which is the same as that of the polycrystalline FeCO3 uPLD target (not shown). Further examination of the crystalline quality of the textured FeCO3 films was done by measuring the rocking curves of the XRD peaks with a four-circle X-ray diffractometer. As shown in Fig. 5, the rocking curve of the (104) XRD peak of the FeCO3 films grown at a substrate temperature of 250 °C shows a width of 0.05° in FWHM, confirming the strong texturing. The rocking curves of the Al2O3 (104) substrates were also measured for comparison (inset in Fig. 5). The rocking-curve width of the (104) XRD peak of a bare Al2O3 substrate is about 0.02° in FWHM. However, with a FeCO3 (104) film deposited on the substrate the rocking curve of the Al2O3 (104) shifts and its width increases to a value of 0.05° (not shown), equal to the (104) XRD peak of the deposited FeCO3 film. This shift and broadening may come from the in-plane lattice mismatch between the FeCO3 film and the Al2O3 substrate [13], which sets a lower limit to the rocking-curve width of the deposited FeCO3 film and the underlying substrate surface layers.
XRD spectra of 2-μm FeCO3 films deposited on Al2O3 (104) substrates at substrate temperatures of (a) 100 °C, (b) 200 °C, (c) 250 °C, and (d) 300 °C. The ablation laser beam for uPLD is of 810-nm central wavelength, \(1\mbox{-J/cm}^{2}\) fluence, 46-fs pulse duration, and on-target spot size. The target-to-substrate distance is 40 mm. The inset in (c) shows a photo of the film grown at 250 °C substrate temperature
Raman spectra of 2-μm FeCO3 films deposited on Al2O3 (104) substrates at substrate temperatures of (a) 250 °C and (b) 300 °C. The other parameters are the same as those in Fig. 3. The inset in (a) shows the Lorentzian fit of the FeCO3 Raman peak in (a)
For the film grown at a substrate temperature of 300 °C the XRD spectrum (Fig. 3(d)) shows a weaker FeCO3 (104) peak with the other peaks remaining absent. This reveals that when grown at this substrate temperature only part of the film possesses a FeCO3 (104) texture and the rest turns amorphous. This is also supported by the Raman spectroscopy measurement (Fig. 4(b)), which shows that one broad peak corresponding to Fe2O3 appears, indicating that part of the film decomposes and thereby produces Fe2O3 amorphous domains in the film.
The strongly textured FeCO3 films were also characterized with Mössbauer spectroscopy. The reasons for using this diagnostic are twofold. Firstly, Mössbauer spectroscopy is a sensitive technique for analyzing crystal structure and crystallinity of a material [14]. Secondly, since the deposited FeCO3 films are intended for application in research of nuclear quantum optics, the characteristics of their Mössbauer spectra can provide direct evidence that the films have the quality required for application in this field. For reference, the Mössbauer spectrum of a polycrystalline 100-μm-thick FeCO3 film produced with the same preparation method as for the uPLD target but without sintering was also measured. The reference sample exhibits a typical Mössbauer spectrum of crystalline FeCO3, in which two distinct peaks are present as a result of quadrupole splitting [4], as shown in Fig. 6(a). Figure 6(b) shows the Mössbauer spectrum of a FeCO3 film produced at a substrate temperature of 250 °C. In amorphous FeCO3 domains 57Fe nuclei at different locations experience different electric field gradients and thus different magnitudes of quadrupole splitting, resulting in an inhomogeneous broadening of the absorption peaks in the Mössbauer spectrum. The resemblance between these two spectra, without broadening of the peaks in the latter compared to the former, supports the absence of amorphous domains in the grown film [15]. This corroborates the conclusion of the Raman spectroscopy measurement, which also rules out the presence of amorphous domains. The observation of these absorption peaks also constitutes the first step towards application in research of nuclear quantum optics based on FeCO3 [4].
Figure 7 shows the surface profiles of the films grown at various substrate temperatures, measured with a surface profiler. As shown, the film surface is smoother for a film grown at a higher substrate temperature. Above a transition temperature of 200–250 °C the film surface becomes flat (with a large-scale surface roughness of \({\approx} 46~\mbox{nm}\) or better) and remains so with further increase of substrate temperature. Such a transition in surface morphology matches with the transition of crystallinity from polycrystalline to strongly textured observed in the XRD spectra. These observations indicate that at a substrate temperature above the transition temperature adatoms can acquire sufficient mobility to diffuse on the deposition surface, resulting in increased crystallographic order [16, 17] and thus a strongly textured film with sub-nanometer flatness (see below). The surface morphology of the strongly textured FeCO3 films was further examined by using SEM and AFM. The results are shown in Fig. 8. Both images reveal that there are micrometer-size particulates on the film surface. The particulates can be ascribed to clusters formed during plume propagation or ejection directly from the uPLD target. In the region without particulates, the AFM image gives a root-mean-square roughness of 0.65 nm. Since the total volume of the particulates is negligible compared to that of the strongly textured film, as is evident from the absence of its effect on the XRD, Raman, and Mössbauer spectra, the film as-grown has the quality required for application in research of nuclear quantum optics, in which only film crystallinity matters.
Surface profiles of 2-μm FeCO3 films deposited on Al2O3 (104) substrates at substrate temperatures of (a) 100 °C, (b) 200 °C, (c) 250 °C, and (d) 300 °C. The other parameters are the same as those in Fig. 3. \(R\) and \(V\) represent the root-mean-square roughness and thickness variation, respectively
(a) SEM and (b) AFM images of a 2-μm FeCO3 film deposited on an Al2O3 (104) substrate at a substrate temperature of 250 °C. The other parameters are the same as those in Fig. 3. \(R\) represents the root-mean-square roughness inside the square region
The dependencies of the crystallinity and surface morphology of grown FeCO3 film on various laser parameters were also studied, with the substrate temperature fixed at 250 °C. The morphology results are summarized in Table 1. The XRD spectra (not shown) indicate that the grown film maintains strongly textured regardless of variation of laser energy fluence, on-target spot area, or pulse duration by a factor of 10. In contrast, with a 10-fold increase in laser fluence or on-target spot area the film acquires a roughness of nearly 100 %, similar to the results with optimal laser parameters but at insufficiently low substrate temperatures. This could be ascribed to the changes in instantaneous deposition flux. By increasing the ablation laser spot size or the fluence, the plume density and thus the instantaneous deposition flux increases. A higher instantaneous deposition flux may lead to a rougher surface by causing adatoms to nucleate rapidly before they migrate to fill in pits on film surface, thereby increasing film roughness. Although a larger laser fluence may result in higher ion momentum in the ablation plume, which should help flatten the film surface via enhancing surface mobility of adatoms [18, 19], it seems that keeping the peak deposition flux sufficiently low is essential for growing an FeCO3 film with excellent surface flatness. Changing the pulse duration from 46 fs to 500 fs does not affect the crystallinity and surface flatness significantly. Because both of the two pulse durations are shorter than the time needed to couple the energy of electronic excitation to lattice vibration (a few picoseconds), the instantaneous deposition flux and ion momentum for the two cases will be comparable, resulting in similar ablation processes.
4 Conclusions
In summary, using PLD, strongly textured FeCO3 (104) films are successfully grown on \(\mathrm{Al}_{2}\mathrm{O}_{3}\) (104) substrates. The crystalline character of the films is reflected by the presence of a single sharp peak in XRD spectrum that corresponds to strong FeCO3 (104) texture and the narrow peak in Raman spectrum that rules out the presence of amorphous domains. In addition, the measured Mössbauer spectrum not only confirms the crystallinity and crystal structure of the deposited FeCO3 films but also demonstrates the feasibility of their application in research of nuclear quantum optics. Furthermore, it is found that the film crystallinity is critically dependent on substrate temperature and substrate type, but relatively insensitive to variation of ablation laser parameters. In contrast, the surface morphology of the grown FeCO3 film is found to be strongly dependent on various laser parameters, and the dependencies indicate that a sufficiently low peak deposition flux is crucial for growing an FeCO3 film with a smooth surface. The thickness of the strongly textured films grown in this demonstration experiment is 2 μm and the thickness variation is \({\le}2.3~{\%}\). Based on the observations in this experiment, growing a much thicker film does not seem to be hindered by any inherent problem. The presence of some micrometer-size particulates does not pose a problem for the above-mentioned application. For other applications that cannot tolerate the presence of particulates, post-machining laser beam can be implemented to alleviate the problem [20, 21]. Based on the results of this experiment, which show that the problem of decarbonation can be overcome with uPLD, it is concluded that uPLD has potential for growing other kinds of carbonate crystalline films. In fact, growing an FeCO3 film is probably the most challenging among all carbonates, because FeCO3 has a relatively low decomposition temperature compared to other carbonates (e.g., CaCO3 at 900 °C).
References
O. Sel, A.V. Radha, K. Dideriksen, A. Navrotsky, Geochim. Cosmochim. Acta 87, 61 (2012)
R. Coussement, Y. Rostovtsev, J. Odeurs, G. Neyens, H. Muramatsu, S. Gheysen, R. Callens, K. Vyvey, G. Kozyreff, P. Mandel, R. Shakhmuratov, O. Kocharovskaya, Phys. Rev. Lett. 89, 107601 (2002)
P. Anisimov, F. Vagizov, Y. Rostovtsev, R. Shakhmuratov, O. Kocharovskaya, J. Mod. Opt. 54, 2595 (2007)
S. Gheysen, J. Odeurs, J. Phys. Condens. Matter 20, 485214 (2008)
K. Byrappa, M. Yoshimura, Handbook of Hydrothermal Technology (William Andrew, Norwich, 2001)
D. Alkaç, Ü. Atalay, Int. J. Miner. Process. 87, 120 (2008)
J. Wang, T. Sakakura, N. Ishizawa, H. Eba, IOP Conf. Ser., Mater. Sci. Eng. 18, 022011 (2011)
D.B. Chrisey, G.K. Huber, Pulsed Laser Deposition of Thin Films (Wiley, New York, 1994)
R. Eason (ed.), Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials (Wiley, New York, 2007)
P.R. Willmott, J.R. Huber, Rev. Mod. Phys. 72, 315 (2000)
O. Albert, S. Roger, Y. Glinec, J.C. Loulergue, J. Etchepare, C. Boulmer-Leborgne, J. Perriére, E. Millon, Appl. Phys. A 76, 319 (2003)
H.-H. Chu, S.-Y. Huang, L.-S. Yang, T.-Y. Chien, Y.-F. Xiao, J.-Y. Lin, C.-H. Lee, S.-Y. Chen, J. Wang, Appl. Phys. B 79, 193 (2004)
U. Pietsch, V. Holy, T. Baumbach, High-Resolution X-Ray Scattering from Thin Films and Multilayers, 2nd edn. (Springer, Berlin, 2004)
Y.-L. Chen, D.-P. Yang, Mössbauer Effect in Lattice Dynamics (Wiley/VCH, Weinheim, 2007)
G.J. Long, D. Hautot, Q.A. Pankhurst, D. Vandormael, F. Grandkean, J.P. Gaspard, V. Briois, T. Hyeon, K.S. Suslick, Phys. Rev. B 57, 10716 (1998)
X. Chen, N.J. Wu, D.L. Ritums, A. Ignatiev, Thin Solid Films 342, 61 (1999)
A. Infortuna, A.S. Harvey, L.J. Gauckler, Adv. Funct. Mater. 18, 127 (2008)
X.L. Tong, D.S. Jiang, L. Liu, Z.M. Liu, M.Z. Luo, Opt. Commun. 270, 356 (2007)
B. Shin, M.J. Aziz, Phys. Rev. B 76, 085431 (2007)
S. Witanachchi, K. Ahmed, P. Sakthivel, P. Mukherjee, Appl. Phys. Lett. 66, 1469 (1995)
E. György, I.N. Mihailescu, M. Kompitsas, A. Giannoudakos, Appl. Surf. Sci. 195, 270 (2002)
Acknowledgements
The work was supported by Academia Sinica Thematic Project and by the National Science Council of Taiwan under Contract No. 98-2112-M-001-012-MY3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yen, CP., Wong, CS., Yeh, CY. et al. Growth of strongly textured \(\mathrm{FeCO}_{3}\) thin films for application in research of nuclear quantum optics with ultrashort-pulsed laser deposition. Appl. Phys. A 115, 671–677 (2014). https://doi.org/10.1007/s00339-013-7848-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-013-7848-3