Abstract
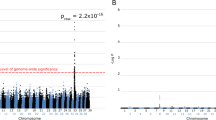
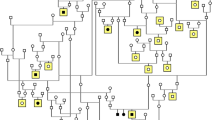
Retinal degeneration (RD) in the Miniature Long Haired Dachshund (MLHD) is a cone-rod dystrophy resulting in eventual blindness in affected individuals. In a previous study, a 44-nucleotide insertion (ins44) in exon 2 of RPGRIP1 was associated with RD. However, results on an extended population of MLHD revealed a variable RD onset age for ins44 homozygous dogs. Further investigations using a genome-wide association study comparing early onset and late onset RD cases identified an age of onset modifying locus for RD, approximately 30 Mb upstream of RPGRIP1 on chr15. In this investigation, target enriched sequencing identified a MAP9 deletion spanning approximately 22 kb associated with early RD onset. Identification of the deletion required correction to the CanFam3.1 genome build as canine MAP9 is part of a historic tandem duplication, resulting in incomplete assembly of this genome region. The deletion breakpoints were identified in MAP9 intron 10 and in a downstream partial MAP9 pseudogene. The fusion of these two genes, which we have called MAP9 EORD (microtubule-associated protein, early onset retinal degeneration), is in frame and is expressed at the RNA level, with the 3′ region containing several predicted deleterious variants. We speculate that MAP9 associates with α-tubulin in the basal body of the cilium. RPGRIP1 is also known to locate to the cilium, where it is closely associated with RPGR. RPGRIP1 mutations also cause redistribution of α-tubulin away from the ciliary region in photoreceptors. Hence, a MAP9 partial deficit is a particularly attractive candidate to synergise with a partial RPGRIP1 deficit to cause a more serious disease.





Similar content being viewed by others
References
Boylan JP, Wright AF (2000) Identification of a novel protein interacting with RPGR. Hum Mol Genet 9(14):2085–2093
Busse C, Barnett KC, Mellersh CS, Adams VJ (2011) Ophthalmic and cone derived electrodiagnostic findings in outbred Miniature Long-haired Dachshunds homozygous for a RPGRIP1 mutation. Vet Ophthalmol 14:146–152
Curtis R, Barnett KC (1993) Progressive retinal atrophy in miniature longhaired dachshund dogs. Br Vet J 149:71–85
Dryja TP, Adams SM, Grimsby JL, McGee TL, Hong DH, Li T, Andreasson S, Berson EL (2001) Null RPGRIP1 alleles in patients with Leber congenital amaurosis. Am J Hum Genet 68:1295–1298
Estrada-Cuzcano A, Roepman R, Cremers FP, den Hollander AI, Mans DA (2012) Non-syndromic retinal ciliopathies: translating gene discovery into therapy. Hum Mol Genet 21:R111–R124
Fontenille L, Rouquier S, Lutfalla G, Giorgi D (2014) Microtubule-associated protein 9 (Map9/Asap) is required for the early steps of zebrafish development. Cell Cycle 13:1101–1114
Gerber S, Perrault I, Hanein S, Barbet F, Ducroq D, Ghazi I, Martin-Coignard D, Leowski C, Homfray T, Dufier JL, Munnich A, Kaplan J, Rozet JM (2001) Complete exon-intron structure of the RPGR-interacting protein (RPGRIP1) gene allows the identification of mutations underlying Leber congenital amaurosis. Eur J Hum Genet 9:561–571
Hong D-H, Yue G, Adamian M, Li T (2001) Retinitis pigmentosa GTPase regulator (RPGR)-interacting protein is stably associated with the photoreceptor ciliary axoneme and anchors RPGR to the connecting cilium. J Biol Chem 276:12091–12099
Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, Maubaret C, Diaz-Font A, MacDonald I, Muzny DM, Wheeler DA, Morgan M, Lewis LR, Logan CV, Tan PL, Beer MA, Inglehearn CF, Lewis RA, Jacobson SG, Bergmann C, Beales PL, Attie-Bitach T, Johnson CA, Otto EA, Bhattacharya SS, Hildebrandt F, Gibbs RA, Koenekoop RK, Swaroop A, Katsanis N (2009) A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet 41:739–745
Kuznetsova T, Iwabe S, Boesze-Battaglia K, Pearce-Kelling S, Chang-Min Y, McDaid K, Miyadera K, Komaromy A, Aguirre GD (2012) Exclusion of RPGRIP1 ins44 from primary causal association with early-onset cone-rod dystrophy in dogs. Invest Ophthalmol Vis Sci 53:5486–5501
Lheriteau E, Libeau L, Stieger K, Deschamps JY, Mendes-Madeira A, Provost N, Lemoine F, Mellersh C, Ellinwood NM, Cherel Y, Moullier P, Rolling F (2009) The RPGRIP1-deficient dog, a promising canine model for gene therapy. Mol Vis 15:349–361
Lheriteau E, Petit L, Weber M, Le Meur G, Deschamps JY, Libeau L, Mendes-Madeira A, Guihal C, Francois A, Guyon R, Provost N, Lemoine F, Papal S, El-Amraoui A, Colle MA, Moullier P, Rolling F (2014) Successful gene therapy in the RPGRIP1-deficient dog: a large model of cone-rod dystrophy. Mol Ther 22:265–277
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760
Liu Q, Tan G, Levenkova N, Li T, Pugh EN Jr, Rux JJ, Speicher DW, Pierce EA (2007) The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics 6:1299–1317
Maddox DM, Ikeda S, Ikeda A, Zhang W, Krebs MP, Nishina PM, Naggert JK (2012) An allele of microtubule-associated protein 1A (Mtap1a) reduces photoreceptor degeneration in Tulp1 and Tub mutant mice. Invest Ophthalmol Vis Sci 53:1663–1669
Markand S, Saul A, Tawfik A, Cui X, Rozen R, Smith SB (2015) Mthfr as a modifier of the retinal phenotype of Crb1 mice. Exp Eye Res 145:164–172
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303
Mellersh CS, Boursnell ME, Pettitt L, Ryder EJ, Holmes NG, Grafham D, Forman OP, Sampson J, Barnett KC, Blanton S, Binns MM, Vaudin M (2006) Canine RPGRIP1 mutation establishes cone-rod dystrophy in miniature longhaired dachshunds as a homologue of human Leber congenital amaurosis. Genomics 88:293–301
Miyadera K, Kato K, Aguirre-Hernandez J, Tokuriki T, Morimoto K, Busse C, Barnett K, Holmes N, Ogawa H, Sasaki N, Mellersh CS, Sargan DR (2009) Phenotypic variation and genotype-phenotype discordance in canine cone-rod dystrophy with an RPGRIP1 mutation. Mol Vis 15:2287–2305
Miyadera K, Brierley I, Aguirre-Hernandez J, Mellersh CS, Sargan DR (2012a) Multiple mechanisms contribute to leakiness of a frameshift mutation in canine cone-rod dystrophy. PLoS ONE 7:e51598
Miyadera K, Kato K, Boursnell M, Mellersh CS, Sargan DR (2012b) Genome-wide association study in RPGRIP1(−/−) dogs identifies a modifier locus that determines the onset of retinal degeneration. Mamm Genome 23:212–223
Narfstrom K, Jeong M, Hyman J, Madsen RW, Bergstrom TF (2012) Assessment of hereditary retinal degeneration in the English springer spaniel dog and disease relationship to an RPGRIP1 mutation. Stem cells Int 2012:685901
Ng PC, Henikoff S (2003) SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res 31:3812–3814
Patil H, Tserentsoodol N, Saha A, Hao Y, Webb M, Ferreira PA (2012) Selective loss of RPGRIP1-dependent ciliary targeting of NPHP4, RPGR and SDCCAG8 underlies the degeneration of photoreceptor neurons. Cell Death Dis 3:e355
Roepman R, Letteboer SJ, Arts HH, van Beersum SE, Lu X, Krieger E, Ferreira PA, Cremers FP (2005) Interaction of nephrocystin-4 and RPGRIP1 is disrupted by nephronophthisis or Leber congenital amaurosis-associated mutations. Proc Natl Acad Sci USA 102:18520–18525
Saffin JM, Venoux M, Prigent C, Espeut J, Poulat F, Giorgi D, Abrieu A, Rouquier S (2005) ASAP, a human microtubule-associated protein required for bipolar spindle assembly and cytokinesis. Proc Natl Acad Sci USA 102:11302–11307
Schon C, Asteriti S, Koch S, Sothilingam V, Garcia Garrido M, Tanimoto N, Herms J, Seeliger MW, Cangiano L, Biel M, Michalakis S (2016) Loss of HCN1 enhances disease progression in mouse models of CNG channel-linked retinitis pigmentosa and achromatopsia. Hum Mol Genet. doi:10.1093/hmg/ddv639
Shu X, Fry AM, Tulloch B, Manson FD, Crabb JW, Khanna H, Faragher AJ, Lennon A, He S, Trojan P, Giessl A, Wolfrum U, Vervoort R, Swaroop A, Wright AF (2005) RPGR ORF15 isoform co-localizes with RPGRIP1 at centrioles and basal bodies and interacts with nucleophosmin. Hum Mol Genet 14(9):1183–1197
Turney C, Chong NH, Alexander RA, Hogg CR, Fleming L, Flack D, Barnett KC, Bird AC, Holder GE, Luthert PJ (2007) Pathological and electrophysiological features of a canine cone-rod dystrophy in the miniature longhaired dachshund. Invest Ophthalmol Vis Sci 48:4240–4249
Venoux M, Basbous J, Berthenet C, Prigent C, Fernandez A, Lamb NJ, Rouquier S (2008) ASAP is a novel substrate of the oncogenic mitotic kinase Aurora-A: phosphorylation on Ser625 is essential to spindle formation and mitosis. Hum Mol Genet 17:215–224
Venturini G, Rose AM, Shah AZ, Bhattacharya SS, Rivolta C (2012) CNOT3 is a modifier of PRPF31 mutations in retinitis pigmentosa with incomplete penetrance. PLoS Genet 8:e1003040
Vollrath D, Yasumura D, Benchorin G, Matthes MT, Feng W, Nguyen NM, Sedano CD, Calton MA, LaVail MM (2015) Tyro3 modulates Mertk-associated retinal degeneration. PLoS Genet 11:e1005723
Xie Y, Wu G, Tang J, Luo R, Patterson J, Liu S, Huang W, He G, Gu S, Li S, Zhou X, Lam TW, Li Y, Xu X, Wong GK, Wang J (2014) SOAPdenovo-Trans: de novo transcriptome assembly with short RNA-Seq reads. Bioinformatics 30:1660–1666
Zhao Y, Hong DH, Pawlyk B, Yue G, Adamian M, Grynberg M, Godzik A, Li T (2003) The retinitis pigmentosa GTPase regulator (RPGR)- interacting protein: subserving RPGR function and participating in disk morphogenesis. Proc Natl Acad Sci USA 100:3965–3970
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
335_2016_9627_MOESM2_ESM.pdf
Sequence read alignments across the deletion breakpoint regions. Definition of the deletion breakpoints was not aided by alignment of targeted enrichment and genome data as poor mapping quality and spurious alignments meant the deletion breakpoint could only be defined to be within a 1666 bp region. Supplementary material 2 (PDF 284 kb)
335_2016_9627_MOESM3_ESM.pdf
Multiple sequence alignments. Multiple sequencing alignments of the de novo assembly of reads generated from the PCR product spanning the deletion to determine the most likely location of the deletion breakpoints. Supplementary material 3 (PDF 96 kb)
335_2016_9627_MOESM6_ESM.pdf
Alignment of massively parallel sequencing reads generated for a Tibetan Terrier and Brittany Spaniel with a deletion across MAP9. Supplementary material 6 (PDF 316 kb)
Rights and permissions
About this article
Cite this article
Forman, O.P., Hitti, R.J., Boursnell, M. et al. Canine genome assembly correction facilitates identification of a MAP9 deletion as a potential age of onset modifier for RPGRIP1-associated canine retinal degeneration. Mamm Genome 27, 237–245 (2016). https://doi.org/10.1007/s00335-016-9627-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-016-9627-x