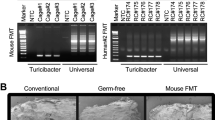
Abstract
The mammalian gastrointestinal (GI) tract is inhabited by over a hundred species of symbiotic bacteria. Differences among individuals in the composition of the GI flora may contribute to variation in in vivo experimental analyses and disease susceptibility. To investigate potential interindividual differences in GI flora composition, we developed real-time quantitative PCR-based assays for the detection of the eight members of the Altered Schaedler Flora (ASF) as representative members of different bacterial niches within the mammalian GI tract. Quantitative and reproducible strain-specific variations in the numbers of the ASF members were observed across 23 different barrier-housed inbred mouse strains, suggesting that the ASF assays can be used as sentinels for changes in GI flora composition. A significant cage effect was also detected. Isogenic mice that cohabited at weaning, whether from the same or different litters, showed little variation in ASF profiles. Conversely, litters split among different cages at weaning showed divergence in ASF profiles after three weeks. Individual ASF profiles, once established, were highly stable over time in the absence of environmental perturbation. Furthermore, cohabitation of different inbred strains maintained most of the interstrain variation in the GI flora, supporting a role of host genetics in determining GI flora composition.





Similar content being viewed by others
References
Akada JK, Ogura K, Dailidiene D, Dailide G, Cheverud JM, et al. (2003) Helicobacter pylori tissue tropism: mouse-colonizing strains can target different gastric niches. Microbiology 149(Pt 7), 1901–1909
Avitsur R, Stark JL, Dhabhar FS, Kramer KA, Sheridan JF (2003) Social experience alters the response to social stress in mice. Brain Behav Immun 17(6), 426–437
Backhed F, Ding H, Wang T, Hooper LV, Koh GY, et al. (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101(44), 15718–15723
Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B (2002) Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab 282(4), E834–842
Cao SG, Wu WC, Han Z, Wang MY (2005) Effects of psychological stress on small intestinal motility and expression of cholecystokinin and vasoactive intestinal polypeptide in plasma and small intestine in mice. World J Gastroenterol 11(5), 737–740
Chow J (2002) Probiotics and prebiotics: A brief overview. J Ren Nutr 12(2), 76–86
Crabbe JC, Wahlsten D (2003) Of mice and their environments. Science 299(5611), 1313–1314
Crabbe JC, Wahlsten D, Dudek BC (1999) Genetics of mouse behavior: interactions with laboratory environment. Science 284(5420), 1670–1672
Crabbe JC, Metten P, Cameron AJ, Wahlsten D (2005) An analysis of the genetics of alcohol intoxication in inbred mice. Neurosci Biobehav Rev 28(8), 785–802
Dewhirst FE, Chien CC, Paster BJ, Ericson RL, Orcutt RP, et al. (1999) Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl Environ Microbiol 65(8), 3287–3292
Dubos R, Savage DC, Schaedler RW (1967) The indigenous flora of the gastrointestinal tract. Dis Colon Rectum 10, 23–34
Falk PG, Hooper LV, Midtvedt T, Gordon JI (1998) Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev 62(4), 1157–1170
Foulon I, Pierard D, Muyldermans G, Vandoorslaer K, Soetens O, et al. (2003) Prevalence of fragilysin gene in Bacteroides fragilis isolates from blood and other extraintestinal samples. J Clin Microbiol 41(9), 4428–4430
Fukui M, Fujino T, Tsutsui K, Maruyama T, Yoshimura H, et al. (2001) The tumor-preventing effect of a mixture of several lactic acid bacteria on 1,2-dimethylhydrazine-induced colon carcinogenesis in mice. Oncol Rep 8(5), 1073–1078
Fuller R, Gibson GR (1997) Modification of the intestinal microflora using probiotics and prebiotics. Scand J Gastroenterol Suppl 222, 28–31
Gustafsson A, Berstad A, Lund-Tonnesen S, Midtvedt T, Norin E (1999) The effect of faecal enema on five microflora-associated characteristics in patients with antibiotic-associated diarrhoea. Scand J Gastroenterol 34(6), 580–586
Hopkins MJ, Macfarlane GT (2002) Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol 51(5), 448–454
Lin MY, Yen CL, Chen SH (1998) Management of lactose maldigestion by consuming milk containing lactobacilli. Dig Dis Sci 43(1), 133–137
Lucky TD (1972) Introduction to microbial ecology. Am J Clin Nutr 25, 1292–1294
Marashi V, Barnekow A, Sachser N (2004) Effects of environmental enrichment on males of a docile inbred strain of mice. Physiol Behav 82(5), 765–776
Nobaek S, Johansson ML, Molin G, Ahrne S, Jeppsson B (2000) Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol 95(5), 1231–1238
Nyberg J, Sandnabba K, Schalkwyk L, Sluyter F (2004) Genetic and environmental (inter)actions in male mouse lines selected for aggressive and nonaggressive behavior. Genes Brain Behav 3(2), 101–109
Orcutt RP, Gianni FJ, Judge RJ (1987) Development of an “Altered Schaedler Flora” for NCI gnotobiotic rodents. Microecol Ther 17, 59
Rolfe RD (2000) The role of probiotic cultures in the control of gastrointestinal health. J Nutr 130(2S Suppl), 396S–402S
Salvin SB, Rabin BS, Neta R (1990) Evaluation of immunologic assays to determine the effects of differential housing on immune reactivity. Brain Behav Immun 4, 180–188
Salzman NH, de Jong H, Paterson Y, Harmsen HJ, Welling GW, et al. (2002) Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology 148(Pt 11), 3651–3660
Sarma-Rupavtarm RB, Ge Z, Schauer DB, Fox JG, Polz MF (2004) Spatial distribution and stability of the eight microbial species of the altered schaedler flora in the mouse gastrointestinal tract. Appl Environ Microbiol 70(5), 2791–2800
Savage DC (1977) Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 31, 107–133
Savage DC (1981) The microbial flora in the gastrointestinal tract. Prog Clin Biol Res 77, 893–908
Savage DC, Dubos R, Schaedler RW (1968) The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med 127, 67–76
Sayegh JF, Kobor G, Lajtha A, Vadasz C (1990) Effects of social isolation and the time of day on testosterone levels in plasma of C57BL/6By and BALB/cBy mice. Steroids 55(2), 79–82
Scislowska-Czarnecka A, Chadzinska M, Pierzchala-Koziec K, Plytycz B (2004) Long-lasting effects of social stress on peritoneal inflammation in some strains of mice. Folia Biol (Krakow) 52(1–2), 97–104
Shanahan F (2004) Host–flora interactions in inflammatory bowel disease. Inflamm Bowel Dis 10(Suppl 1), S16–24
Tang X, Orchard SM, Sanford LD (2002) Home cage activity and behavioral performance in inbred and hybrid mice. Behav Brain Res 136(2), 555–569
Todar K (2002) Todar’s Online Textbook of Bacteriology (Madison, WI: Department of Bacteriology, University of Wisconsin–Madison)
Toivanen P, Vaahtovuo J, Eerola E (2001) Influence of major histocompatibility complex on bacterial composition of fecal flora. Infect Immun 69(4), 2372–2377
Tuohy KM, Probert HM, Smejkal CW, Gibson GR (2003) Using probiotics and rebiotics to improve gut health. Drug Discov Today 8(15), 692–700
Wahlsten D, Metten P, Phillips TJ, Boehm SL 2nd, Burkhart-Kasch S, et al. (2003a) Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol 54(1), 283–311
Wahlsten D, Rustay NR, Metten P, Crabbe JC (2003b) In search of a better mouse test. Trends Neurosci 26(3), 132–136
Wang SX, Wu WC (2005) Effects of psychological stress on small intestinal motility and bacteria and mucosa in mice. World J Gastroenterol 11(13), 2016–2021
Wilson JR, Limaye AP (2004) Risk factors for mortality in patients with anaerobic bacteremia. Eur J Clin Microbiol Infect Dis 23(4), 310–316
Zhou RH, Tsutsumi K, Nakano S (1997) Effects of isolation housing and timing of drug administration on amikacin kinetics in mice. Zhongguo Yao Li Xue Bao 18(4), 303–305
Zoetendal EG, Ben-Amor K, Akkermans AD, Abee T, de Vos WM (2001) DNA isolation protocols affect the detection limit of PCR approaches of bacteria in samples from the human gastrointestinal tract. Syst Appl Microbiol 24(3), 405–410
Acknowledgments
The authors thank Dr. H.S. Kim for assistance in preparing the qPCR primers and dual-labeled probes, and Maureen Bower, Jerri Shaw, Doug Behnke, and Gary Grimm of the UNC CGIBD Gnotobiotic Core for assistance in maintaining germfree mice. This work was supported by a Pilot and Feasibility grant from the UNC GCIBD, supported by NIH grant DK34987, by a Research Supplement for Underrepresented Minorities to NIH grant CA79869 and by NIH grant CA84239.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deloris Alexander, A., Orcutt, R.P., Henry, J.C. et al. Quantitative PCR assays for mouse enteric flora reveal strain-dependent differences in composition that are influenced by the microenvironment. Mamm Genome 17, 1093–1104 (2006). https://doi.org/10.1007/s00335-006-0063-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-006-0063-1