Abstract
Objectives
To examine whether post-chemoradiotherapy (CRT) DCE-MRI can identify rectal cancer patients with pathologic complete response (pCR).
Methods
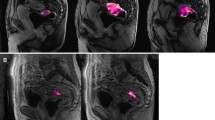
From a rectal cancer surgery database 2007–2014, 61 consecutive patients that met the following inclusion criteria were selected for analysis: (1) stage II/III primary rectal adenocarcinoma; (2) received CRT; (3) underwent surgery (4); underwent rectal DCE-MRI on a 1.5-T MRI scanner. Two experienced radiologists, in consensus, drew regions of interest (ROI) on the sagittal DCE-MRI image in the tumour bed. These were exported from ImageJ to in-house Matlab code for modelling using the Tofts model. K trans, K ep and v e values were compared to pathological response.
Results
Of the 61 initial patients, 37 had data considered adequate for fitting to obtain perfusion parameters. Among the 13 men and 24 women, median age 53 years, there were 8 pCR (22 %). K trans could not distinguish patients with pCR. For patients with 90 % or greater response, mean K trans and K ep values were statistically significant (p = 0.032 and 0.027, respectively). Using a cutoff value of K trans = 0.25 min−1, the AUC was 0.71.
Conclusion
K trans could be used to identify patients with 90 % or more response to chemoradiotherapy for rectal cancer with an AUC of 0.7.
Key Points
• Chemoradiotherapy for rectal cancer causes decreased blood flow and permeability in the tumour bed.
• Lower values of blood flow and permeability correlate with good tumour response.
• K trans of 0.25min −1 best identifies patients with ≥90 % response with AUC 0.71




Similar content being viewed by others
References
Gollub MJ, Gultekin DH, Akin O, Do RK, Fuqua JL 3rd, Gonen M et al (2012) Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol 22:821–831
Goh V, Padhani AR, Rasheed S (2007) Functional imaging of colorectal cancer angiogenesis. Lancet Oncol 8:245–255
Hötker AM, Tarlinton L, Mazaheri Y, Woo KM, Gönen M, Saltz LB, Goodman KA, Garcia-Aguilar J, Gollub MJ (2016) Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: a comparison of morphological, volumetric and functional MRI parameters. Eur Radiol. doi:10.1007/s00330-016-4283-9
de Lussanet QG, Backes WH, Griffioen AW, Padhani AR, Baeten CI, van Baardwijk A et al (2005) Dynamic contrast-enhanced magnetic resonance imaging of radiation therapy-induced microcirculation changes in rectal cancer. Int J Radiat Oncol Biol Phys 63:1309–1315
Atkin G, Taylor NJ, Daley FM, Stirling JJ, Richman P, Glynne-Jones R et al (2006) Dynamic contrast-enhanced magnetic resonance imaging is a poor measure of rectal cancer angiogenesis. Br J Surg 93:992–1000
Kremser C, Trieb T, Rudisch A, Judmaier W, de Vries A (2007) Dynamic T(1) mapping predicts outcome of chemoradiation therapy in primary rectal carcinoma: sequence implementation and data analysis. J Magn Reson Imaging 26:662–671
Sahani DV, Kalva SP, Hamberg LM, Hahn PF, Willett CG, Saini S et al (2005) Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology 234:785–792
Dinter DJ, Horisberger K, Zechmann C, Wenz F, Brade J, Willeke F et al (2009) Can dynamic MR imaging predict response in patients with rectal cancer undergoing cetuximab-based neoadjuvant chemoradiation? Onkologie 32:86–93
de Vries A, Griebel J, Kremser C, Judmaier W, Gneiting T, Debbage P et al (2000) Monitoring of tumor microcirculation during fractionated radiation therapy in patients with rectal carcinoma: preliminary results and implications for therapy. Radiology 217:385–391
Devries AF, Griebel J, Kremser C, Judmaier W, Gneiting T, Kreczy A et al (2001) Tumor microcirculation evaluated by dynamic magnetic resonance imaging predicts therapy outcome for primary rectal carcinoma. Cancer Res 61:2513–2516
Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R et al (2008) Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 72:99–107
Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R et al (2005) Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 23:8688–8696
Kim SH, Lee JM, Gupta SN, Han JK, Choi BI (2014) Dynamic contrast-enhanced MRI to evaluate the therapeutic response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer. J Magn Reson Imaging 40:730–737
Oberholzer K, Menig M, Pohlmann A, Junginger T, Heintz A, Kreft A et al (2013) Rectal cancer: assessment of response to neoadjuvant chemoradiation by dynamic contrast-enhanced MRI. J Magn Reson Imaging 38:119–126
Lim JS, Kim D, Baek SE, Myoung S, Choi J, Shin SJ et al (2012) Perfusion MRI for the prediction of treatment response after preoperative chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 22:1693–1700
George ML, Dzik-Jurasz AS, Padhani AR, Brown G, Tait DM, Eccles SA et al (2001) Non-invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. Br J Surg 88:1628–1636
Yeo DM, Oh SN, Jung CK, Lee MA, Oh ST, Rha SE et al (2015) Correlation of dynamic contrast-enhanced MRI perfusion parameters with angiogenesis and biologic aggressiveness of rectal cancer: preliminary results. J Magn Reson Imaging 41:474–480
Tong T, Sun Y, Gollub MJ, Peng W, Cai S, Zhang Z et al (2015) Dynamic contrast-enhanced MRI: use in predicting pathological complete response to neoadjuvant chemoradiation in locally advanced rectal cancer. J Magn Reson Imaging 42:673–680
Intven M, Reerink O, Philippens ME (2015) Dynamic contrast enhanced MR imaging for rectal cancer response assessment after neo-adjuvant chemoradiation. J Magn Reson Imaging 41:1646–1653
Intven M, Monninkhof EM, Reerink O, Philippens ME (2015) Combined T2w volumetry, DW-MRI and DCE-MRI for response assessment after neo-adjuvant chemoradiation in locally advanced rectal cancer. Acta Oncol 54:1729–1736
Martens MH, Subhani S, Heijnen LA, Lambregts DM, Buijsen J, Maas M et al (2015) Can perfusion MRI predict response to preoperative treatment in rectal cancer? Radiother Oncol 114:218–223
Kalff V, Ware R, Heriot A, Chao M, Drummond E, Hicks RJ (2009) Radiation changes do not interfere with post-chemoradiation restaging of patients with rectal cancer by FDG PET/CT before curative surgical therapy. Int J Radiat Oncol Biol Phys 74:60–66
Kalff V, Duong C, Drummond EG, Matthews JP, Hicks RJ (2006) Findings on 18F-FDG PET scans after neoadjuvant chemoradiation provides prognostic stratification in patients with locally advanced rectal carcinoma subsequently treated by radical surgery. J Nucl Med 47:14–22
Maas M, Lambregts DM, Nelemans PJ, Heijnen LA, Martens MH, Leijtens JW et al (2015) Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ-saving treatment. Ann Surg Oncol 22:3873–3880
Martens MH, Lambregts DMJ, Papnikolaou N, Alefantinou S, Maas M, Manikis GC et al (2016) Magnetization transfer imaging to assess tumor response after chemoradiotherapy in rectal cancer. Eur Radiol 26:390–397
Habr-Gama A, Gama-Rodrigues J, Sao Juliao GP, Proscurshim I, Sabbagh C, Lynn PB et al (2014) Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 88:822–828
Smith JD, Ruby JA, Goodman KA, Saltz LB, Guillem JG, Weiser MR et al (2012) Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg 256:965–972
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Koh TS, Bisdas S, Koh DM, Thng CH (2011) Fundamentals of tracer kinetics for dynamic contrast-enhanced MRI. J Magn Reson Imaging 34:1262–1276
Parker GJ, Roberts C, Macdonald A, Buonaccorsi GA, Cheung S, Buckley DL et al (2006) Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magn Reson Med 56:993–1000
Shia J, Guillem JG, Moore HG, Tickoo SK, Qin J, Ruo L et al (2004) Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol 28:215–223
Trakarnsanga A, Gonen M, Shia J, Nash GM, Temple LK, Guillem JG et al (2014) Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. doi:10.1093/jnci/dju248
Mandard AM, Dalibard F, Mandard JC et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73:2680–2686
Intven M, Reerink O, Philippens ME (2013) Diffusion-weighted MRI in locally advanced rectal cancer: pathological response prediction after neo-adjuvant radiochemotherapy. Strahlenther Onkol 189:117–122
Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M et al (2011) Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol 18:2224–2231
Lambrecht M, Vandecaveye V, De Keyzer F, Roels S, Penninckx F, Van Cutsem E et al (2012) Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: preliminary results. Int J Radiat Oncol Biol Phys 82:863–870
Kim SH, Lee JY, Lee JM, Han JK, Choi BI (2011) Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur Radiol 21:987–995
Lambregts DM, Beets GL, Maas M, Curvo-Semedo L, Kessels AG, Thywissen T et al (2011) Tumour ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol 21:2567–2574
Petrillo A, Fusco R, Petrillo M, Granata V (2015) Standardized index of shape (SIS): a quantitative DCE-MRI parameter to discriminate responders by non-responders after neoadjuvant therapy in LARC. Eur Radiol 25:1935–1945
Acknowledgements
The scientific guarantor of this publication is Marc J. Gollub, MD. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This study has received funding by National Institutes of Health (P30 CA008748). Junting Zheng and Mithat Gonen kindly provided statistical advice for this manuscript. Both authors have significant statistical expertise. Institutional review board approval was obtained. Written informed consent was waived by the institutional review board. We thank the research team of Dr. Regina Beets-Tan for patient T1-value data provided.
Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Marc J. Gollub and Tong Tong contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gollub, M.J., Tong, T., Weiser, M. et al. Limited accuracy of DCE-MRI in identification of pathological complete responders after chemoradiotherapy treatment for rectal cancer. Eur Radiol 27, 1605–1612 (2017). https://doi.org/10.1007/s00330-016-4493-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4493-1