Abstract
Objectives
We hypothesized that non-contrast-enhanced PETRA (pointwise encoding time reduction with radial acquisition) MR (magnetic resonance) sequencing could be an alternative to unenhanced computed tomography (CT) in assessing cystic fibrosis (CF) lung structural alterations, as well as compared agreements and concordances with those of conventional T1-weighted and T2-weighted sequences.
Material and methods
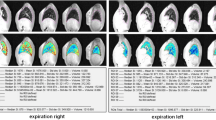
Thirty consecutive CF patients completed both CT and MRI the same day. No contrast injection was used. Agreement in identifying structural alterations was evaluated at the segmental level using a kappa test. Intraclass correlation coefficients (ICC) and Bland-Altman analysis were used to assess concordances and reproducibility in Helbich-Bhalla disease severity scoring.
Results
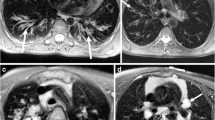
Agreement between PETRA and CT was higher than that of T1- or T2-weighted sequences, notably in assessing the segmental presence of bronchiectasis (Kappa = 0.83; 0.51; 0.49, respectively). The concordance in Helbich-Bhalla scores was very good using PETRA (ICC = 0.97), independently from its magnitude (mean difference (MD) = -0.3 [-2.8; 2.2]), whereas scoring was underestimated using both conventional T1 and T2 sequences (MD = -3.6 [-7.4; 0.1]) and MD = -4.6 [-8.2; -1.0], respectively). Intra- and interobserver reproducibility were very good for all imaging modalities (ICC = 0.86-0.98).
Conclusion
PETRA showed higher agreement in describing CF lung morphological changes than that of conventional sequences, whereas the Helbich-Bhalla scoring matched closely with that of CT.
Key Points
• Spatial resolution of lung MRI is limited using non-ultra-short TE MRI technique
• Ultra-short echo time (UTE) technique enables submillimeter 3D-MRI of airways
• 3D-UTE MRI shows very good concordance with CT in assessing cystic fibrosis
• Radiation-free 3D-UTE MRI enables the Helbich-Bhalla scoring without a need for contrast injection




Similar content being viewed by others
Abbreviations
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- 3D:
-
Three-dimensional
- 2D:
-
Two-dimensional
- UTE:
-
Ultra-short echo time
- GRE:
-
Gradient echo
- PETRA:
-
Pointwise encoding time reduction with radial acquisition
- CF:
-
Cystic fibrosis
- ICC:
-
Intraclass correlation coefficient
- MD:
-
Mean deviation (N mm)3 = N × N × N mm3
References
Jain M, Goss CH (2014) Update in cystic fibrosis 2013. Am J Respir Crit Care Med 189:1181–1186
Kuehn BM (2014) Progress in treating cystic fibrosis means that many patients may now reach midlife and beyond. JAMA 312:1182–1183
Loeve M, Krestin GP, Rosenfeld M, de Bruijne M, Stick SM, Tiddens HA (2013) Chest computed tomography: a validated surrogate endpoint of cystic fibrosis lung disease? Eur Respir J 42:844–857
Bhalla M, Turcios N, Aponte V et al (1991) Cystic fibrosis: scoring system with thin-section CT. Radiology 179:783–788
de Jong PA, Lindblad A, Rubin L et al (2006) Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax 61:80–85
O'Connor OJ, Vandeleur M, McGarrigle AM et al (2010) Development of low-dose protocols for thin-section CT assessment of cystic fibrosis in pediatric patients. Radiology 257:820–829
Leuraud K, Richardson DB, Cardis E et al (2015) Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol. doi:10.1016/S2352-3026(15)00094-0
Wielputz MO, Mall MA (2015) Imaging modalities in cystic fibrosis: emerging role of MRI. Curr Opin Pulm Med 21:609–616
Altes TA, Eichinger M, Puderbach M (2007) Magnetic resonance imaging of the lung in cystic fibrosis. Proc Am Thorac Soc 4:321–327
Thomson LK, Thomson PC, Kingsmore DB et al (2014) Diagnosing nephrogenic systemic fibrosis in the post-FDA restriction era. J Magn Reson Imaging. doi:10.1002/jmri.24664
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Tepper LA, Ciet P, Caudri D, Quittner AL, Utens EM, Tiddens HA (2015) Validating chest MRI to detect and monitor cystic fibrosis lung disease in a pediatric cohort. Pediatr Pulmonol. doi:10.1002/ppul.23328
Eichinger M, Optazaite DE, Kopp-Schneider A et al (2012) Morphologic and functional scoring of cystic fibrosis lung disease using MRI. Eur J Radiol 81:1321–1329
Puderbach M, Eichinger M, Gahr J et al (2007) Proton MRI appearance of cystic fibrosis: comparison to CT. Eur Radiol 17:716–724
Renz DM, Scholz O, Bottcher J et al (2015) Comparison between magnetic resonance imaging and computed tomography of the lung in patients with cystic fibrosis with regard to clinical, laboratory, and pulmonary functional parameters. Invest Radiol. doi:10.1097/RLI.0000000000000178
Ciet P, Serra G, Bertolo S et al (2015) Assessment of CF lung disease using motion corrected PROPELLER MRI: a comparison with CT. Eur Radiol. doi:10.1007/s00330-015-3850-9
Sileo C, Corvol H, Boelle PY, Blondiaux E, Clement A, Ducou Le Pointe H (2014) HRCT and MRI of the lung in children with cystic fibrosis: comparison of different scoring systems. J Cyst Fibros 13:198–204
Johnson KM, Fain SB, Schiebler ML, Nagle S (2013) Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med 70:1241–1250
Dournes G, Grodzki D, Macey J et al (2015) Quiet submillimeter MR imaging of the lung Is feasible with a PETRA sequence at 1.5 T. Radiology. doi:10.1148/radiol.15141655:141655
Ohno Y, Koyama H, Yoshikawa T et al (2015) Pulmonary high-resolution ultrashort TE MR imaging: comparison with thin-section standard- and low-dose computed tomography for the assessment of pulmonary parenchyma diseases. J Magn Reson Imaging. doi:10.1002/jmri.25008
Helbich TH, Heinz-Peer G, Eichler I et al (1999) Cystic fibrosis: CT assessment of lung involvement in children and adults. Radiology 213:537–544
de Jong PA, Ottink MD, Robben SG et al (2004) Pulmonary disease assessment in cystic fibrosis: comparison of CT scoring systems and value of bronchial and arterial dimension measurements. Radiology 231:434–439
Kerem E, Conway S, Elborn S, Heijerman H, Consensus C (2005) Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros 4:7–26
The Cystic Fibrosis Foundation Center Committee and Guidelines Subcommittee (1990). Cystic Fibrosis Foundation guidelines for patient services, evaluation, and monitoring in cystic fibrosis centers.. Am J Dis Child 144:1311–1312
Wielputz MO, Heussel CP, Herth FJ, Kauczor HU (2014) Radiological diagnosis in lung disease: factoring treatment options into the choice of diagnostic modality. Dtsch Arztebl Int 111:181–187
Haute Autorité de Santé (2006) Mucoviscidose. Protocole National de Diagnostic et de Soins pour une Maladie Rare. Available via http://www.has-sante.fr/portail/jcms/c_464719/fr/ald-n18-mucoviscidose. Accessed 20 november 2015
Loeve M, Lequin MH, de Bruijne M et al (2009) Cystic fibrosis: are volumetric ultra-low-dose expiratory CT scans sufficient for monitoring related lung disease? Radiology 253:223–229
Lee ES, Lee JM, Yu MH et al (2014) High spatial resolution, respiratory-gated, t1-weighted magnetic resonance imaging of the liver and the biliary tract during the hepatobiliary phase of gadoxetic acid-enhanced magnetic resonance imaging. J Comput Assist Tomogr 38:360–366
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246:697–722
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Burris NS, Johnson KM, Larson PE et al (2015) Detection of small pulmonary nodules with ultrashort echo time sequences in oncology patients by using a PET/MR system. Radiology. doi:10.1148/radiol.2015150489:150489
Bannas P, Bell LC, Johnson KM et al (2015) Pulmonary embolism detection with three-dimensional ultrashort echo time MR imaging: experimental study in canines. Radiology. doi:10.1148/radiol.2015150606:150606
Grodzki DM, Jakob PM, Heismann B (2012) Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med 67:510–518
Bankier AA, De Maertelaer V, Keyzer C, Gevenois PA (1999) Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology 211:851–858
Ernst CW, Basten IA, Ilsen B et al (2014) Pulmonary disease in cystic fibrosis: assessment with chest CT at chest radiography dose levels. Radiology 273:597–605
Rosenow T, Oudraad MC, Murray CP et al (2015) PRAGMA-CF. A quantitative structural lung disease computed tomography outcome in young children with cystic fibrosis. Am J Respir Crit Care Med 191:1158–1165
Fink C, Puderbach M, Biederer J et al (2007) Lung MRI at 1.5 and 3 Tesla: observer preference study and lesion contrast using five different pulse sequences. Invest Radiol 42:377–383
Yan C, Tan X, Wei Q et al (2015) Lung MRI of invasive fungal infection at 3 Tesla: evaluation of five different pulse sequences and comparison with multidetector computed tomography (MDCT). Eur Radiol 25:550–557
Wielputz MO, Puderbach M, Kopp-Schneider A et al (2014) Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med 189:956–965
Maisonneuve P, Marshall BC, Knapp EA, Lowenfels AB (2013) Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst 105:122–129
Buzan MT, Eichinger M, Kreuter M et al (2015) T2 mapping of CT remodelling patterns in interstitial lung disease. Eur Radiol 25:3167–3174
Bryant M, Ley S, Eberhardt R et al (2015) Assessment of the relationship between morphological emphysema phenotype and corresponding pulmonary perfusion pattern on a segmental level. Eur Radiol 25:72–80
Acknowledgments
The scientific guarantor of this publication is François Laurent. The authors thank Mr. David Grodzki from Siemens Healthcare Company for technical support. This study has received funding by the Laboratory of Excellence TRAIL, ANR-10-LABX-57. One of the authors (Professor Patrick Berger) has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
Criteria of cystic fibrosis severity according to the Helbich Scoring System (XLSX 14 kb)
Supplemental Table 2
Artifact assessment using CT and MRI (XLSX 14 kb)
Rights and permissions
About this article
Cite this article
Dournes, G., Menut, F., Macey, J. et al. Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Eur Radiol 26, 3811–3820 (2016). https://doi.org/10.1007/s00330-016-4218-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4218-5