Abstract
Objectives
The aim of this study was to investigate spinal cord structure in patients with cervical spondylosis where conventional MRI fails to reveal spinal cord damage.
Methods
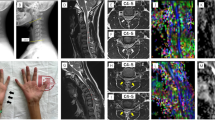
We performed a cross-sectional study of patients with cervical spondylosis without conventional MRI findings of spinal cord damage and healthy controls. Subjects were studied using spinal diffusion tensor imaging (DTI), precision grip and foot force-tracking tasks, and a clinical examination including assessment of neurological signs. A regional analysis of lateral and medial spinal white matter across multiple cervical levels (C1–C5) was performed.
Results
DTI revealed reduced fractional anisotropy (FA) and increased radial diffusivity (RD) in the lateral spinal cord at the level of greatest compression (lowest Pavlov ratio) in patients (p < 0.05). Patients with spondylosis had greater error and longer release duration in both grip and foot force-tracking. Similar spinal cord deficits were present in patients without neurological signs. Increased error in grip and foot tracking (low accuracy) correlated with increased RD in the lateral spinal cord at the level of greatest compression (p ≤ 0.01).
Conclusions
Spinal DTI can detect subtle spinal cord damage of functional relevance in cervical spondylosis, even in patients without signs on conventional T2-imaging and without neurological signs.
Key Points
• DTI reveals spinal cord changes in cervical spondylosis with few symptoms
• DTI changes were present despite normal spinal cord on conventional MRI
• DTI parameters correlated with force control accuracy in hand and foot
• Spinal DTI is a promising technique for patients with cervical spondylosis




Similar content being viewed by others
Abbreviations
- AD:
-
Axial diffusivity
- ADC:
-
Apparent diffusion coefficient
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- N:
-
Newton
- RD:
-
Radial diffusivity
- ROI:
-
Region of interest
- SD:
-
Standard deviation
References
Brain WR, Northfield D, Wilkinson M (1952) The neurological manifestations of cervical spondylosis. Brain 75:187–225
Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG (2015) Degenerative cervical myelopathy: epidemiology, genetics and pathogenesis. Spine (Phila Pa 1976). doi:10.1097/BRS.0000000000000913
Holly LT (2009) Management of cervical spondylotic myelopathy with insights from metabolic imaging of the spinal cord and brain. Curr Opin Neurol 22:575–581
Chikuda H, Seichi A, Takeshita K et al (2010) Correlation between pyramidal signs and the severity of cervical myelopathy. Eur Spine J 19:1684–1689
Morio Y, Teshima R, Nagashima H, Nawata K, Yamasaki D, Nanjo Y (2001) Correlation between operative outcomes of cervical compression myelopathy and MRI of the spinal cord. Spine (Phila Pa 1976) 26:1238–1245
Kerkovský M, Bednarík J, Dušek L et al (2012) Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine (Phila Pa 1976) 37:48–56
Nair G, Carew JD, Usher S, Lu D, Hu XP, Benatar M (2010) Diffusion tensor imaging reveals regional differences in the cervical spinal cord in amyotrophic lateral sclerosis. Neuroimage 53:576–583
Naismith RT, Xu J, Klawiter EC et al (2013) Spinal cord tract diffusion tensor imaging reveals disability substrate in demyelinating disease. Neurology 80:2201–2209
Stroman PW, Wheeler-Kingshott C, Bacon M et al (2014) The current state-of-the-art of spinal cord imaging: methods. Neuroimage 84:1070–1081
Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D (2005) MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol 26:1587–1594
Mamata H, Jolesz FA, Maier SE (2005) Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes. J Magn Reson Imaging 22:38–43
Demir A, Ries M, Moonen CT et al (2003) Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology 229:37–43
Banaszek A, Bladowska J, Szewczyk P, Podgórski P, Sąsiadek M (2014) Usefulness of diffusion tensor MR imaging in the assessment of intramedullary changes of the cervical spinal cord in different stages of degenerative spine disease. Eur Spine J 23:1523–1530
Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M (2013) Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am J Neuroradiol 34:471–478
Ellingson BM, Salamon N, Grinstead JW, Holly LT (2014) Diffusion tensor imaging predicts functional impairment in mild-to-moderate cervical spondylotic myelopathy. Spine J 14:2589–2597
Ellingson BM, Salamon N, Woodworth DC, Holly LT (2015) Correlation between degree of subvoxel spinal cord compression measured with super-resolution tract density imaging and neurological impairment in cervical spondylotic myelopathy. J Neurosurg Spine 6:1–8
Vitzthum HE, Dalitz K (2007) Analysis of five specific scores for cervical spondylogenic myelopathy. Eur Spine J 16:2096–2103
Amirjani N, Ashworth NL, Gordon T, Edwards DC, Chan KM (2007) Normative values and the effects of age, gender, and handedness on the Moberg pick-up test. Muscle Nerve 35:788–792
Lindberg PG, Feydy A, Maier MA (2010) White matter organization in cervical spinal cord relates differently to age and control of grip force in healthy subjects. J Neurosci 30:4102–4109
Roche N, Bussel B, Maier MA, Katz R, Lindberg P (2011) Impact of precision grip tasks on cervical spinal network excitability in humans. J Physiol 589:3545–3558
Lindberg P, Ody C, Feydy A, Maier MA (2009) Precision in isometric precision grip force is reduced in middle-aged adults. Exp Brain Res 193:213–224
Lindberg PG, Bensmail D, Bussel B, Maier MA, Feydy A (2011) Wallerian degeneration in lateral cervical spinal cord detected with diffusion tensor imaging in four chronic stroke patients. J Neuroimaging 21:44–48
Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17:1429–1436
Pavlov H, Torg JS, Robie B, Jahre C (1987) Cervical spinal stenosis: determination with vertebral body ratio method. Radiology 164:771–775
Ciccarelli O, Wheeler-Kingshott CA, McLean MA et al (2007) Spinal cord spectroscopy and diffusion-based tractography to assess acute disability in multiple sclerosis. Brain 130:2220–2231
Van Hecke W, Leemans A, Sijbers J, Vandervliet E, Van Goethem J, Parizel PM (2008) A tracking-based diffusion tensor imaging segmentation method for the detection of diffusion-related changes of the cervical spinal cord with aging. J Magn Reson Imaging 27:978–991
Karadimas SK, Moon ES, Yu WR et al (2013) A novel experimental model of cervical spondylotic myelopathy (CSM) to facilitate translational research. Neurobiol Dis 54:43–58
Nathan PW, Smith MC, Deacon P (1990) The corticospinal tracts in man. Course and location of fibres at different segmental levels. Brain 113:303–324
Ogino H, Tada K, Okada K et al (1983) Canal diameter, anteroposterior compression ratio, and spondylotic myelopathy of the cervical spine. Spine (Phila Pa 1976) 8:1–15
Simó M, Szirmai I, Arányi Z (2004) Superior sensitivity of motor over somatosensory evoked potentials in the diagnosis of cervical spondylotic myelopathy. Eur J Neurol 11:621–626
Blight AR (1991) Morphometric analysis of a model of spinal cord injury in guinea pigs, with behavioral evidence of delayed secondary pathology. J Neurol Sci 103:156–171
Quencer RM, Bunge RP, Egnor M et al (1992) Acute traumatic central cord syndrome: MRI-pathological correlations. Neuroradiology 34:85–94
Lemon RN (2008) Descending pathways in motor control. Annu Rev Neurosci 31:195–218
Zijdewind I, Thomas CK (2003) Motor unit firing during and after voluntary contractions of human thenar muscles weakened by spinal cord injury. J Neurophysiol 89:2065–2071
Yoshikawa M, Doita M, Okamoto K, Manabe M, Sha N, Kurosaka M (2008) Impaired postural stability in patients with cervical myelopathy: evaluation by computerized static stabilometry. Spine (Phila Pa 1976) 33:E460–E464
Olindo S, Signate A, Richech A et al (2008) Quantitative assessment of hand disability by the Nine-Hole-Peg test (9-HPT) in cervical spondylotic myelopathy. J Neurol Neurosurg Psychiatry 79:965–967
Freund P, Schneider T, Nagy Z et al (2012) Degeneration of the injured cervical cord is associated with remote changes in corticospinal tract integrity and upper limb impairment. PLoS One 7, e51729
Hatem SM, Attal N, Ducreux D et al (2010) Clinical, functional and structural determinants of central pain in syringomyelia. Brain 133:3409–3422
Koskinen E, Brander A, Hakulinen U et al (2013) Assessing the state of chronic spinal cord injury using diffusion tensor imaging. J Neurotrauma 30:1587–1595
Ducreux D, Lepeintre JF, Fillard P, Loureiro C, Tadié M, Lasjaunias P (2006) MR diffusion tensor imaging and fiber tracking in 5 spinal cord astrocytomas. AJNR Am J Neuroradiol 27:214–216
Agosta F, Lagana M, Valsasina P et al (2007) Evidence for cervical cord tissue disorganisation with aging by diffusion tensor MRI. Neuroimage 36:728–735
Cui JL, Wen CY, Hu Y, Mak KC, Mak KH, Luk KD (2011) Orientation entropy analysis of diffusion tensor in healthy and myelopathic spinal cord. Neuroimage 58:1028–1033
Acknowledgments
This study was in part supported by grants to Påvel Lindberg of Hjärnfonden (the Swedish Brain Foundation) and of the Institut pour la Recherche sur la Moelle Epinière et l'Encéphale (IRME, Paris). The authors thank URC-CIC Paris Centre for implementation and monitoring of the study. The scientific guarantor of this publication is Marc A. Maier. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: cross-sectional study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindberg, P.G., Sanchez, K., Ozcan, F. et al. Correlation of force control with regional spinal DTI in patients with cervical spondylosis without signs of spinal cord injury on conventional MRI. Eur Radiol 26, 733–742 (2016). https://doi.org/10.1007/s00330-015-3876-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3876-z